当前位置:
X-MOL 学术
›
J. Am. Chem. Soc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Carbon Kinetic Isotope Effects and the Mechanisms of Acid-Catalyzed Decarboxylation of 2,4-Dimethoxybenzoic Acid and CO2 Incorporation into 1,3-Dimethoxybenzene
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2017-10-13 , DOI: 10.1021/jacs.7b07504 Adelle A. Vandersteen 1 , Graeme W. Howe 1 , Barbara Sherwood Lollar 1 , Ronald Kluger 1
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2017-10-13 , DOI: 10.1021/jacs.7b07504 Adelle A. Vandersteen 1 , Graeme W. Howe 1 , Barbara Sherwood Lollar 1 , Ronald Kluger 1
Affiliation
|
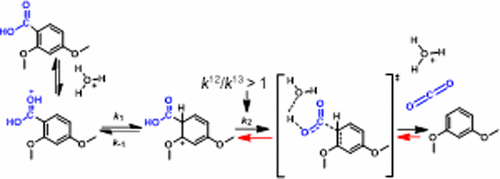
The rate of decarboxylation of 2,4-dimethoxybenzoic acid (1) is accelerated in parallel to the extent that the carboxyl group acquires a second proton (1H+). However, the conjugate acid would resist C-C bond breaking as that would lead to formation of doubly protonated CO2. An alternative via formation of a higher-energy protonated phenyl tautomer (2H+) prior to C-C bond breaking would produce protonated CO2, an energetically inaccessible species that can be avoided by transfer of the carboxyl proton to water in the same step. Headspace sampling of CO2 that evolves in the acid-catalyzed process and analysis by GC-IRMS gives a smaller than expected value of 1.022 for the carbon kinetic isotope (CKIE), k12/k13. While this value establishes that C-C cleavage is part of the rate-determining process, intrinsic CKIEs for decarboxylation reactions are typically greater than 1.03. Computational analysis of the C-C bond cleavage from 2H+ gives an intrinsic CKIE of 1.051 and suggests two partially rate-determining steps in the decarboxylation of 1: transfer of the second carboxyl proton to the adjacent phenyl carbon and C-C cleavage in which the carboxyl proton is also transferred to water. Applying the principle of microscopic reversibility to fixation of CO2 in acidic solutions reveals the importance of proton transfers to both carbon and oxygen in the overall fixation process.
中文翻译:

碳动力学同位素效应及2,4-二甲氧基苯甲酸酸催化脱羧和CO2掺入1,3-二甲氧基苯的机理
2,4-二甲氧基苯甲酸 (1) 的脱羧速率随着羧基获得第二个质子 (1H+) 的程度而加快。然而,共轭酸会阻止 CC 键断裂,因为这会导致双质子化 CO2 的形成。在 CC 键断裂之前通过形成更高能量的质子化苯基互变异构体 (2H+) 的替代方法会产生质子化 CO2,这是一种能量上难以接近的物质,可以通过在同一步骤中将羧基质子转移到水中来避免。对酸催化过程中产生的 CO2 进行顶空采样并通过 GC-IRMS 进行分析,得出的碳动力学同位素 (CKIE) k12/k13 小于预期值 1.022。虽然该值表明 CC 裂解是速率决定过程的一部分,脱羧反应的内在 CKIE 通常大于 1.03。对 2H+ 的 CC 键裂解的计算分析给出了 1.051 的内在 CKIE,并表明 1 的脱羧中有两个部分决定速率的步骤:将第二个羧基质子转移到相邻的苯基碳和 CC 裂解,其中羧基质子也是转移到水中。将微观可逆性原理应用于酸性溶液中 CO2 的固定,揭示了质子在整个固定过程中向碳和氧转移的重要性。第二个羧基质子转移到相邻的苯基碳和 CC 裂解,其中羧基质子也转移到水中。将微观可逆性原理应用于酸性溶液中 CO2 的固定,揭示了质子在整个固定过程中向碳和氧转移的重要性。第二个羧基质子转移到相邻的苯基碳和 CC 裂解,其中羧基质子也转移到水中。将微观可逆性原理应用于酸性溶液中 CO2 的固定,揭示了质子在整个固定过程中向碳和氧转移的重要性。
更新日期:2017-10-13
中文翻译:

碳动力学同位素效应及2,4-二甲氧基苯甲酸酸催化脱羧和CO2掺入1,3-二甲氧基苯的机理
2,4-二甲氧基苯甲酸 (1) 的脱羧速率随着羧基获得第二个质子 (1H+) 的程度而加快。然而,共轭酸会阻止 CC 键断裂,因为这会导致双质子化 CO2 的形成。在 CC 键断裂之前通过形成更高能量的质子化苯基互变异构体 (2H+) 的替代方法会产生质子化 CO2,这是一种能量上难以接近的物质,可以通过在同一步骤中将羧基质子转移到水中来避免。对酸催化过程中产生的 CO2 进行顶空采样并通过 GC-IRMS 进行分析,得出的碳动力学同位素 (CKIE) k12/k13 小于预期值 1.022。虽然该值表明 CC 裂解是速率决定过程的一部分,脱羧反应的内在 CKIE 通常大于 1.03。对 2H+ 的 CC 键裂解的计算分析给出了 1.051 的内在 CKIE,并表明 1 的脱羧中有两个部分决定速率的步骤:将第二个羧基质子转移到相邻的苯基碳和 CC 裂解,其中羧基质子也是转移到水中。将微观可逆性原理应用于酸性溶液中 CO2 的固定,揭示了质子在整个固定过程中向碳和氧转移的重要性。第二个羧基质子转移到相邻的苯基碳和 CC 裂解,其中羧基质子也转移到水中。将微观可逆性原理应用于酸性溶液中 CO2 的固定,揭示了质子在整个固定过程中向碳和氧转移的重要性。第二个羧基质子转移到相邻的苯基碳和 CC 裂解,其中羧基质子也转移到水中。将微观可逆性原理应用于酸性溶液中 CO2 的固定,揭示了质子在整个固定过程中向碳和氧转移的重要性。

































 京公网安备 11010802027423号
京公网安备 11010802027423号