JAMA Psychiatry ( IF 22.5 ) Pub Date : 2017-10-01 , DOI: 10.1001/jamapsychiatry.2017.2432 Cosima Locher 1 , Helen Koechlin 2 , Sean R. Zion 3 , Christoph Werner 1 , Daniel S. Pine 4 , Irving Kirsch 5 , Ronald C. Kessler 6 , Joe Kossowsky 7
|
Importance Depressive disorders (DDs), anxiety disorders (ADs), obsessive-compulsive disorder (OCD), and posttraumatic stress disorder (PTSD) are common mental disorders in children and adolescents.
Objective To examine the relative efficacy and safety of selective serotonin reuptake inhibitors (SSRIs), serotonin-norepinephrine reuptake inhibitors (SNRIs), and placebo for the treatment of DD, AD, OCD, and PTSD in children and adolescents.
Data Sources PubMed, EMBASE, PsycINFO, Web of Science, and Cochrane Database from inception through August 7, 2016.
Study Selection Published and unpublished randomized clinical trials of SSRIs or SNRIs in youths with DD, AD, OCD, or PTSD were included. Trials using other antidepressants (eg, tricyclic antidepressants, monoamine oxidase inhibitors) were excluded.
Data Extraction and Synthesis Effect sizes, calculated as standardized mean differences (Hedges g) and risk ratios (RRs) for adverse events, were assessed in a random-effects model.
Main Outcomes and Measures Primary outcomes, as defined by authors on preintervention and postintervention data, mean change data, and adverse event data, were extracted independently by multiple observers following PRISMA guidelines.
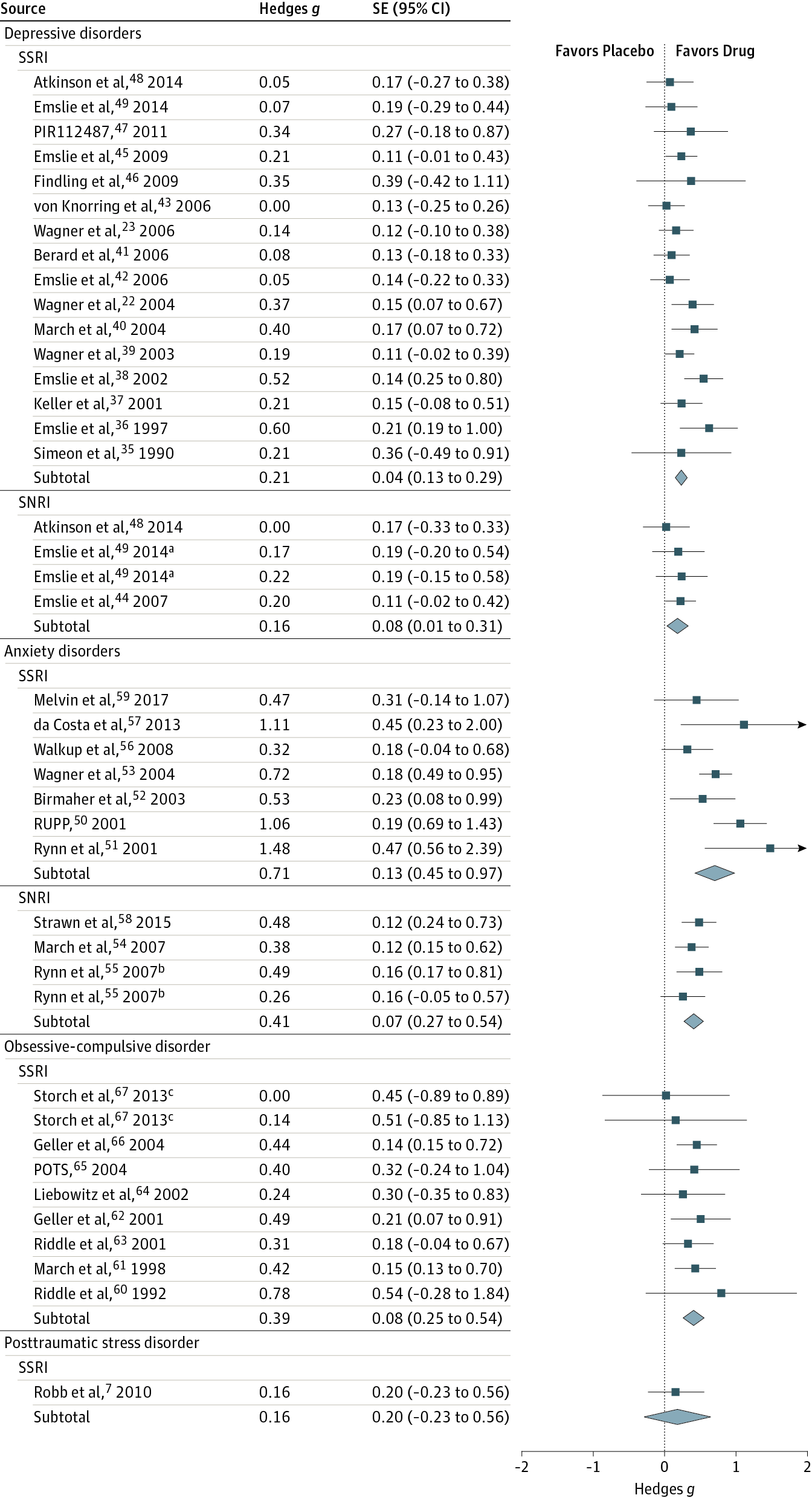
Results Thirty-six trials were eligible, including 6778 participants (3484 [51.4%] female; mean [SD] age, 12.9 [5.1] years); 17 studies for DD, 10 for AD, 8 for OCD, and 1 for PTSD. Analysis showed that SSRIs and SNRIs were significantly more beneficial compared with placebo, yielding a small effect size (g = 0.32; 95% CI, 0.25-0.40; P < .001). Anxiety disorder (g = 0.56; 95% CI, 0.40-0.72; P < .001) showed significantly larger between-group effect sizes than DD (g = 0.20; 95% CI, 0.13-0.27; P < .001). This difference was driven primarily by the placebo response: patients with DD exhibited significantly larger placebo responses (g = 1.57; 95% CI, 1.36-1.78; P < .001) compared with those with AD (g = 1.03; 95% CI, 0.84-1.21; P < .001). The SSRIs produced a relatively large effect size for ADs (g = 0.71; 95% CI, 0.45-0.97; P < .001). Compared with participants receiving placebo, patients receiving an antidepressant reported significantly more treatment-emergent adverse events (RR, 1.07; 95% CI, 1.01-1.12; P = .01 or RR, 1.49; 95% CI, 1.22-1.82; P < .001, depending on the reporting method), severe adverse events (RR, 1.76; 95% CI, 1.34-2.32; P < .001), and study discontinuation due to adverse events (RR, 1.79; 95% CI, 1.38-2.32; P < .001).
Conclusions and Relevance Compared with placebo, SSRIs and SNRIs are more beneficial than placebo in children and adolescents; however, the benefit is small and disorder specific, yielding a larger drug-placebo difference for AD than for other conditions. Response to placebo is large, especially in DD. Severe adverse events are significantly more common with SSRIs and SNRIs than placebo.
中文翻译:

选择性5-羟色胺再摄取抑制剂,5-羟色胺-去甲肾上腺素再摄取抑制剂和安慰剂对儿童和青少年常见精神疾病的疗效和安全性系统评价和荟萃分析
重要性 抑郁症(DDs),焦虑症(ADs),强迫症(OCD)和创伤后应激障碍(PTSD)是儿童和青少年的常见精神障碍。
目的 探讨选择性5-羟色胺再摄取抑制剂(SSRIs),5-羟色胺去甲肾上腺素再摄取抑制剂(SNRIs)和安慰剂在儿童和青少年中DD,AD,OCD和PTSD的相对疗效和安全性。
从开始到2016年8月7日的数据源PubMed,EMBASE,PsycINFO,Web of Science和Cochrane数据库。
研究选择包括已 发表和未发表的DD,AD,OCD或PTSD青年SSRI或SNRI的随机临床试验。排除使用其他抗抑郁药(例如三环抗抑郁药,单胺氧化酶抑制剂)的试验。
在随机效应模型中评估数据提取和综合效应的大小,以不良事件的标准化平均差异(Hedges g)和风险比(RRs)计算。
主要结局和措施 主要作者根据干预前和干预后数据,平均变化数据和不良事件数据定义的主要结果由PRISMA指南的多个观察员独立提取。
结果 共有36项试验符合条件,其中包括6778名参与者(3484 [51.4%]名女性;平均[SD]年龄为12.9 [5.1]岁);有3名受试者为研究对象。DD的17个研究,AD的10个研究,OCD的8个研究和PTSD的1个研究。分析表明,与安慰剂相比,SSRI和SNRI明显更有益,产生的效应较小(g = 0.32; 95%CI,0.25-0.40;P <.001)。焦虑症(g = 0.56; 95%CI,0.40-0.72; P <.001)与DD(g = 0.20; 95%CI,0.13-0.27; P <.001)相比,组间效应大小显着更大。这种差异主要是由安慰剂反应引起的:DD患者表现出明显更大的安慰剂反应(g = 1.57; 95%CI,1.36-1.78;P <.001)与AD相比(g = 1.03; 95%CI,0.84-1.21; P <.001)。SSRI对AD产生相对较大的效应大小(g = 0.71; 95%CI,0.45-0.97; P <.001)。与接受安慰剂的参与者相比,接受抗抑郁药的患者报告的治疗后不良事件明显增加(RR,1.07; 95%CI,1.01-1.12;P = 0.01或RR,1.49; 95%CI,1.22-1.82;P < .001,取决于报告方法),严重不良事件(RR,1.76; 95%CI,1.34-2.32;P <.001),以及因不良事件导致的研究中断(RR,1.79; 95%CI,1.38- 2.32; P <.001)。
结论和相关性 与安慰剂相比,SSRI和SNRI对儿童和青少年的益处要大于安慰剂。但是,其益处小且针对特定疾病,与其他情况相比,AD产生的药物安慰剂差异更大。对安慰剂的反应很大,尤其是在DD中。与安慰剂相比,SSRI和SNRI严重不良事件的发生率明显更高。


















































 京公网安备 11010802027423号
京公网安备 11010802027423号