当前位置:
X-MOL 学术
›
ACS Catal.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Spinel-Structured ZnCr2O4 with Excess Zn Is the Active ZnO/Cr2O3 Catalyst for High-Temperature Methanol Synthesis
ACS Catalysis ( IF 11.3 ) Pub Date : 2017-10-06 00:00:00 , DOI: 10.1021/acscatal.7b01822 Huiqing Song 1 , Daniel Laudenschleger 1 , John J. Carey 2 , Holger Ruland 1 , Michael Nolan 2 , Martin Muhler 1
ACS Catalysis ( IF 11.3 ) Pub Date : 2017-10-06 00:00:00 , DOI: 10.1021/acscatal.7b01822 Huiqing Song 1 , Daniel Laudenschleger 1 , John J. Carey 2 , Holger Ruland 1 , Michael Nolan 2 , Martin Muhler 1
Affiliation
|
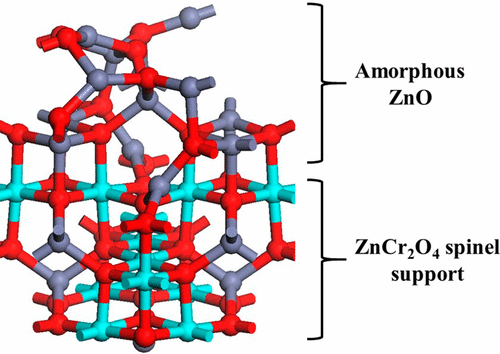
A series of ZnO/Cr2O3 catalysts with different Zn:Cr ratios was prepared by coprecipitation at a constant pH of 7 and applied in methanol synthesis at 260–300 °C and 60 bar. The X-ray diffraction (XRD) results showed that the calcined catalysts with ratios from 65:35 to 55:45 consist of ZnCr2O4 spinel with a low degree of crystallinity. For catalysts with Zn:Cr ratios smaller than 1, the formation of chromates was observed in agreement with temperature-programmed reduction results. Raman and XRD results did not provide evidence for the presence of segregated ZnO, indicating the existence of Zn-rich nonstoichiometric Zn–Cr spinel in the calcined catalyst. The catalyst with Zn:Cr = 65:35 exhibits the best performance in methanol synthesis. The Zn:Cr ratio of this catalyst corresponds to that of the Zn4Cr2(OH)12CO3 precursor with hydrotalcite-like structure obtained by coprecipitation, which is converted during calcination into a nonstoichiometric Zn–Cr spinel with an optimum amount of oxygen vacancies resulting in high activity in methanol synthesis. Density functional theory calculations are used to examine the formation of oxygen vacancies and to measure the reducibility of the methanol synthesis catalysts. Doping Cr into bulk and the (10–10) surface of ZnO does not enhance the reducibility of ZnO, confirming that Cr:ZnO cannot be the active phase. The (100) surface of the ZnCr2O4 spinel has a favorable oxygen vacancy formation energy of 1.58 eV. Doping this surface with excess Zn charge-balanced by oxygen vacancies to give a 60% Zn content yields a catalyst composed of an amorphous ZnO layer supported on the spinel with high reducibility, confirming this as the active phase for the methanol synthesis catalyst.
中文翻译:

尖晶石结构的ZnCr 2 O 4和过量的Zn是高温合成甲醇的活性ZnO / Cr 2 O 3催化剂
通过在恒定pH值为7的条件下共沉淀制备了一系列具有不同Zn:Cr比的ZnO / Cr 2 O 3催化剂,并将其用于260–300°C和60 bar的甲醇合成中。X射线衍射(XRD)结果表明,煅烧催化剂中ZnCr 2 O 4的比例为65:35至55:45。尖晶石结晶度低。对于Zn:Cr比率小于1的催化剂,观察到铬酸盐的形成与程序升温还原结果一致。拉曼和XRD结果并未提供ZnO偏析的证据,表明煅烧催化剂中存在富锌的非化学计量Zn-Cr尖晶石。Zn:Cr = 65:35的催化剂在甲醇合成中表现出最好的性能。该催化剂的Zn:Cr比对应于Zn 4 Cr 2(OH)12 CO 3。通过共沉淀获得的具有类似水滑石结构的前驱体,在煅烧过程中将其转化为非化学计量的Zn-Cr尖晶石,具有最佳的氧空位,从而在甲醇合成中具有很高的活性。密度泛函理论计算用于检查氧空位的形成并测量甲醇合成催化剂的还原性。将Cr掺杂到块体中和ZnO的(10-10)表面不会增强ZnO的可还原性,这证明Cr:ZnO不能为活性相。ZnCr 2 O 4的(100)表面尖晶石具有1.58 eV的有利氧空位形成能。用过量的锌电荷掺杂该表面,该过量的锌电荷通过氧空位来平衡,以提供60%的Zn含量,得到的催化剂由负载在尖晶石上的无定形ZnO层组成,具有高还原性,证实了它是甲醇合成催化剂的活性相。
更新日期:2017-10-06
中文翻译:

尖晶石结构的ZnCr 2 O 4和过量的Zn是高温合成甲醇的活性ZnO / Cr 2 O 3催化剂
通过在恒定pH值为7的条件下共沉淀制备了一系列具有不同Zn:Cr比的ZnO / Cr 2 O 3催化剂,并将其用于260–300°C和60 bar的甲醇合成中。X射线衍射(XRD)结果表明,煅烧催化剂中ZnCr 2 O 4的比例为65:35至55:45。尖晶石结晶度低。对于Zn:Cr比率小于1的催化剂,观察到铬酸盐的形成与程序升温还原结果一致。拉曼和XRD结果并未提供ZnO偏析的证据,表明煅烧催化剂中存在富锌的非化学计量Zn-Cr尖晶石。Zn:Cr = 65:35的催化剂在甲醇合成中表现出最好的性能。该催化剂的Zn:Cr比对应于Zn 4 Cr 2(OH)12 CO 3。通过共沉淀获得的具有类似水滑石结构的前驱体,在煅烧过程中将其转化为非化学计量的Zn-Cr尖晶石,具有最佳的氧空位,从而在甲醇合成中具有很高的活性。密度泛函理论计算用于检查氧空位的形成并测量甲醇合成催化剂的还原性。将Cr掺杂到块体中和ZnO的(10-10)表面不会增强ZnO的可还原性,这证明Cr:ZnO不能为活性相。ZnCr 2 O 4的(100)表面尖晶石具有1.58 eV的有利氧空位形成能。用过量的锌电荷掺杂该表面,该过量的锌电荷通过氧空位来平衡,以提供60%的Zn含量,得到的催化剂由负载在尖晶石上的无定形ZnO层组成,具有高还原性,证实了它是甲醇合成催化剂的活性相。































 京公网安备 11010802027423号
京公网安备 11010802027423号