当前位置:
X-MOL 学术
›
J. Am. Chem. Soc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Structure–Function Studies of Artemisia tridentata Farnesyl Diphosphate Synthase and Chrysanthemyl Diphosphate Synthase by Site-Directed Mutagenesis and Morphogenesis
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2017-10-05 00:00:00 , DOI: 10.1021/jacs.7b07608 J. Scott Lee 1 , Jian-Jung Pan 1 , Gurusankar Ramamoorthy 1 , C. Dale Poulter 1
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2017-10-05 00:00:00 , DOI: 10.1021/jacs.7b07608 J. Scott Lee 1 , Jian-Jung Pan 1 , Gurusankar Ramamoorthy 1 , C. Dale Poulter 1
Affiliation
|
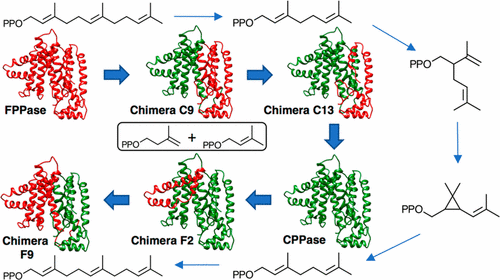
The amino acid sequences of farnesyl diphosphate synthase (FPPase) and chrysanthemyl diphosphate synthase (CPPase) from Artemisia tridentata ssp. Spiciformis, minus their chloroplast targeting regions, are 71% identical and 90% similar. FPPase efficiently and selectively synthesizes the “regular” sesquiterpenoid farnesyl diphosphate (FPP) by coupling isopentenyl diphosphate (IPP) to dimethylallyl diphosphate (DMAPP) and then to geranyl diphosphate (GPP). In contrast, CPPase is an inefficient promiscuous enzyme, which synthesizes the “irregular” monoterpenes chrysanthemyl diphosphate (CPP), lavandulyl diphosphate (LPP), and trace quantities of maconelliyl diphosphate (MPP) from two molecules of DMAPP, and couples IPP to DMAPP to give GPP. A. tridentata FPPase and CPPase belong to the chain elongation protein family (PF00348), a subgroup of the terpenoid synthase superfamily (CL0613) whose members have a characteristic α terpene synthase α-helical fold. The active sites of A. tridentata FPPase and CPPase are located within a six-helix bundle containing amino acids 53 to 241. The two enzymes were metamorphosed into one another by sequentially replacing the loops and helices of the six-helix bundle from enzyme with those from the other. Chain elongation was the dominant activity during the N-terminal to C-terminal metamorphosis of FPPase to CPPase, with product selectivity gradually switching from FPP to GPP, until replacement of the final α-helix, whereupon cyclopropanation and branching activity competed with chain elongation. During the corresponding metamorphosis of CPPase to FPPase, cyclopropanation and branching activities were lost upon replacement of the first helix in the six-helix bundle. Mutations of active site residues in CPPase to the corresponding amino acids in FPPase enhanced chain-elongation activity, while similar mutations in the active site of FPPase failed to significantly promote formation of significant amounts of irregular monoterpenes. Our results indicate that CPPase, a promiscuous enzyme, is more plastic toward acquiring new activities, whereas FPPase is more resistant. Mutations of residues outside of the α terpene synthase fold are important for acquisition of FPPase activity for synthesis of CPP, LPP, and MPP.
中文翻译:

通过定点诱变和形态发生对三蒿蒿法磷酸二磷酸合酶和菊花基磷酸二氢合酶的结构-功能研究
蒿(Artemisia tridentata ssp。)的法呢基二磷酸合酶(FPPase)和菊花基二磷酸合酶(CPPase)的氨基酸序列。螺旋藻减去叶绿体靶向区域后,具有71%的相同性和90%的相似性。FPPase通过将异戊烯基二磷酸酯(IPP)偶联至二甲基烯丙基二磷酸酯(DMAPP),然后偶联至香叶基二磷酸酯(GPP),有效并选择性地合成“常规”倍半萜烯类法呢基二磷酸酯(FPP)。相比之下,CPPase是一种效率低下的混杂酶,它可以从两个DMAPP分子中合成“不规则”的单萜菊二磷酸菊酯(CPP),lavandulyl diphosphate(LPP)和痕量的壬烯基二磷酸酯(MPP),并将IPP与DMAPP偶联到给GPP。三齿FPPase和CPPase属于链延长蛋白家族(PF00348),是萜类合酶超家族(CL0613)的一个子组,其成员具有特征性的α萜烯合酶α-螺旋折叠。A. tridentata的活性部位FPPase和CPPase位于包含53到241位氨基酸的六螺旋束中。通过依次用来自另一酶的六螺旋束的环和螺旋顺序替换来自另一酶的六螺旋束的环和螺旋,使这两种酶相互转化。链延长是FPPase到CPPase的N端到C端变态过程中的主要活性,产物选择性逐渐从FPP切换到GPP,直到最终的α-螺旋被取代,然后环丙烷化和支化活性与链延长竞争。在从CPPase到FPPase的相应变态过程中,替换六螺旋束中的第一个螺旋时,环丙烷化和分支活性丧失。CPPase中活性位点残基突变为FPPase中相应的氨基酸增强了链延伸活性,而FPPase活性位点的相似突变却不能显着促进大量不规则单萜的形成。我们的结果表明,混杂酶CPPase对获得新活性更具可塑性,而FPPase具有更强的抗性。α萜烯合酶折叠以外的残基突变对于获得CPP,LPP和MPP合成所需的FPPase活性很重要。
更新日期:2017-10-05
中文翻译:

通过定点诱变和形态发生对三蒿蒿法磷酸二磷酸合酶和菊花基磷酸二氢合酶的结构-功能研究
蒿(Artemisia tridentata ssp。)的法呢基二磷酸合酶(FPPase)和菊花基二磷酸合酶(CPPase)的氨基酸序列。螺旋藻减去叶绿体靶向区域后,具有71%的相同性和90%的相似性。FPPase通过将异戊烯基二磷酸酯(IPP)偶联至二甲基烯丙基二磷酸酯(DMAPP),然后偶联至香叶基二磷酸酯(GPP),有效并选择性地合成“常规”倍半萜烯类法呢基二磷酸酯(FPP)。相比之下,CPPase是一种效率低下的混杂酶,它可以从两个DMAPP分子中合成“不规则”的单萜菊二磷酸菊酯(CPP),lavandulyl diphosphate(LPP)和痕量的壬烯基二磷酸酯(MPP),并将IPP与DMAPP偶联到给GPP。三齿FPPase和CPPase属于链延长蛋白家族(PF00348),是萜类合酶超家族(CL0613)的一个子组,其成员具有特征性的α萜烯合酶α-螺旋折叠。A. tridentata的活性部位FPPase和CPPase位于包含53到241位氨基酸的六螺旋束中。通过依次用来自另一酶的六螺旋束的环和螺旋顺序替换来自另一酶的六螺旋束的环和螺旋,使这两种酶相互转化。链延长是FPPase到CPPase的N端到C端变态过程中的主要活性,产物选择性逐渐从FPP切换到GPP,直到最终的α-螺旋被取代,然后环丙烷化和支化活性与链延长竞争。在从CPPase到FPPase的相应变态过程中,替换六螺旋束中的第一个螺旋时,环丙烷化和分支活性丧失。CPPase中活性位点残基突变为FPPase中相应的氨基酸增强了链延伸活性,而FPPase活性位点的相似突变却不能显着促进大量不规则单萜的形成。我们的结果表明,混杂酶CPPase对获得新活性更具可塑性,而FPPase具有更强的抗性。α萜烯合酶折叠以外的残基突变对于获得CPP,LPP和MPP合成所需的FPPase活性很重要。































 京公网安备 11010802027423号
京公网安备 11010802027423号