当前位置:
X-MOL 学术
›
Environ. Sci. Technol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Role of Calcium in the Coagulation of NOM with Ferric Chloride
Environmental Science & Technology ( IF 10.8 ) Pub Date : 2017-10-04 00:00:00 , DOI: 10.1021/acs.est.7b02038 Christina C. Davis 1 , Marc Edwards 1
Environmental Science & Technology ( IF 10.8 ) Pub Date : 2017-10-04 00:00:00 , DOI: 10.1021/acs.est.7b02038 Christina C. Davis 1 , Marc Edwards 1
Affiliation
|
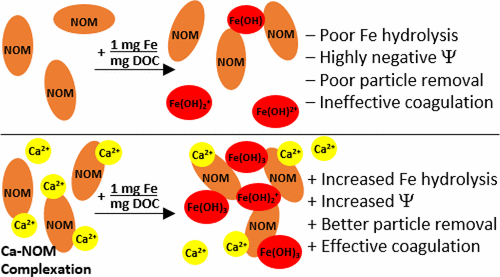
Natural organic matter (NOM) is capable of interfering with Fe hydrolysis and influencing the size, morphology, and identity of Fe precipitates. Conversely, Ca2+ raises surface potential and increases the size and aggregation of Fe precipitates, leading to more effective coagulation and widening the pH range of water treatment. Experiments and modeling were conducted to investigate the significance of the Fe/NOM ratio and the presence of Ca2+ in coagulation. At the high Fe/NOM ratio, sufficient or excess Fe was available for NOM removal, and coagulation proceeded according to expectations based upon the literature. At the low Fe/NOM ratio, however, NOM inhibited Fe hydrolysis, reduced zeta potential, and suppressed the formation of filterable Fe flocs, thereby interfering with NOM removal. In these dose-limited systems without Ca2+, complexation of Fe species by NOM appears to be the mechanism by which coagulation is disrupted. Equilibrating NOM with 1 mM Ca2+ in dose-limited systems prior to dosing with FeCl3 increased Fe hydrolysis and zeta potential, decreased the fraction of colloidal Fe, and improved NOM removal. In systems with Ca2+, data and modeling indicate that Ca2+ complexation by NOM neutralizes some of the negative organic charge and minimizes Fe complexation, making Fe species available for hydrolysis and effective coagulation. This finding represents an important advance in understanding not only how Ca2+ may improve coagulation outcomes, but also in predicting the conditions under which Ca2+ may prove beneficial.
中文翻译:

钙在氯化铁与NOM混凝中的作用
天然有机物(NOM)能够干扰Fe的水解并影响Fe沉淀物的大小,形态和特性。相反,Ca 2+可以提高表面电势,并增加Fe沉淀物的大小和聚集,从而导致更有效的凝结并扩大水处理的pH范围。进行实验和建模以研究Fe / NOM比和Ca 2+的存在的意义在凝血中。在高的Fe / NOM比下,有足够或过量的Fe可用于NOM去除,并且根据文献,凝结根据预期进行。然而,在低Fe / NOM比时,NOM抑制了Fe的水解,降低了Zeta电位,并抑制了可过滤的Fe絮凝物的形成,从而干扰了NOM的去除。在这些没有Ca 2+的剂量受限系统中,NOM对Fe物种的络合似乎是凝血被破坏的机制。在用FeCl 3给药之前,在剂量限制的系统中用1 mM Ca 2+平衡NOM可以增加Fe的水解和Zeta电位,降低胶态Fe的分数,并改善NOM的去除。在具有Ca 2+的系统中,数据和模型表明,NOM的Ca 2+络合作用可中和一些负有机电荷并使Fe络合作用最小化,从而使Fe物种可用于水解和有效凝结。这一发现代表着重要的进展,不仅可以理解Ca 2+如何改善凝血结果,而且可以预测Ca 2+可能有益的条件。
更新日期:2017-10-04
中文翻译:

钙在氯化铁与NOM混凝中的作用
天然有机物(NOM)能够干扰Fe的水解并影响Fe沉淀物的大小,形态和特性。相反,Ca 2+可以提高表面电势,并增加Fe沉淀物的大小和聚集,从而导致更有效的凝结并扩大水处理的pH范围。进行实验和建模以研究Fe / NOM比和Ca 2+的存在的意义在凝血中。在高的Fe / NOM比下,有足够或过量的Fe可用于NOM去除,并且根据文献,凝结根据预期进行。然而,在低Fe / NOM比时,NOM抑制了Fe的水解,降低了Zeta电位,并抑制了可过滤的Fe絮凝物的形成,从而干扰了NOM的去除。在这些没有Ca 2+的剂量受限系统中,NOM对Fe物种的络合似乎是凝血被破坏的机制。在用FeCl 3给药之前,在剂量限制的系统中用1 mM Ca 2+平衡NOM可以增加Fe的水解和Zeta电位,降低胶态Fe的分数,并改善NOM的去除。在具有Ca 2+的系统中,数据和模型表明,NOM的Ca 2+络合作用可中和一些负有机电荷并使Fe络合作用最小化,从而使Fe物种可用于水解和有效凝结。这一发现代表着重要的进展,不仅可以理解Ca 2+如何改善凝血结果,而且可以预测Ca 2+可能有益的条件。





















































 京公网安备 11010802027423号
京公网安备 11010802027423号