当前位置:
X-MOL 学术
›
J. Am. Chem. Soc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Synergic “Click” Boronate/Thiosemicarbazone System for Fast and Irreversible Bioorthogonal Conjugation in Live Cells
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2017-10-02 00:00:00 , DOI: 10.1021/jacs.7b08693
Burcin Akgun 1 , Caishun Li 1 , Yubin Hao 1 , Gareth Lambkin 1 , Ratmir Derda 1 , Dennis G. Hall 1
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2017-10-02 00:00:00 , DOI: 10.1021/jacs.7b08693
Burcin Akgun 1 , Caishun Li 1 , Yubin Hao 1 , Gareth Lambkin 1 , Ratmir Derda 1 , Dennis G. Hall 1
Affiliation
|
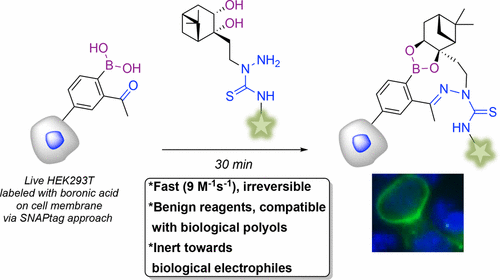
Fast, high-yielding, and selective bioorthogonal “click” reactions employing nontoxic reagents are in high demand for their great utility in the conjugation of biomolecules in live cells. Although a number of click reactions were developed for this purpose, many are associated with drawbacks and limitations that justify the development of alternative systems for both single- or dual-labeling applications. Recent reports have highlighted the potential of boronic ester formation as a bioorthogonal click reaction between abiotic boronic acids and diols. Boronic ester formation is a fast dehydrative process; however it is intrinsically reversible in aqueous medium. We designed and optimized a synergic system based on two bifunctional reagents, a thiosemicarbazide-functionalized nopoldiol and an ortho-acetyl arylboronic acid. Both reagents were shown to be chemically stable and nontoxic to HEK293T cells at concentrations as high as 50 μM. The resulting boronate/thiosemicarbazone adduct is a medium-sized ring that forms rapidly and irreversibly without any catalyst at low μM concentrations, in neutral buffer, with a rate constant of 9 M–1 s–1 as measured by NMR spectroscopy. Control experiments in the presence of competing boronic acids showed no crossover side-products and confirmed the stability and lack of reversibility of the boronate/thiosemicarbazone conjugates. Formation of the conjugates is not affected by the presence of biological diols such as fructose, glucose, and catechol, and the thiosemicarbazide-functionalized nopoldiol is inert to aldehyde electrophiles of the sort found on protein-bound glyoxylyl units. The suitability of this system in the cell-surface labeling of live cells was demonstrated using a SNAP-tag approach to install the boronic acid reagent onto the extracellular domain of the Beta-2 adrenergic receptor in HEK293T cells, followed by incubation with the optimal thiosemicarbazide-functionalized nopoldiol reagent labeled with fluorescein dye. Successful visualization by fluorescence microscopy was possible with a reagent concentration as low as 10 μM, thus confirming the potential of this system in biological applications.
中文翻译:

协同“点击”硼酸/硫代氨基脲系统在活细胞中快速和不可逆的生物正交偶联
对于使用无毒试剂的快速,高产量和选择性的生物正交“点击”反应,在活细胞中生物分子的缀合方面具有巨大的实用性,对此有很高的要求。尽管为此目的开发了许多单击反应,但许多单击反应都与缺点和局限有关,这些缺点和局限性证明了开发用于单标签或双标签应用程序的替代系统的合理性。最近的报道强调了硼酸酯形成的潜力,这是非生物硼酸和二醇之间的生物正交点击反应。硼酸酯的形成是一个快速的脱水过程。然而,它在水性介质中本质上是可逆的。我们设计和优化了基于两种双功能试剂,一种硫代氨基脲官能化的壬二醇和邻位双酚的协同系统。-乙酰基芳基硼酸。两种试剂在浓度高达50μM时对HEK293T细胞均具有化学稳定性和无毒性。所得的硼酸酯/硫代半碳zone加合物是一个中等大小的环,在中性缓冲液中,在低μM浓度下没有任何催化剂的情况下,快速且不可逆地形成,速率常数为9 M –1 s –1通过NMR光谱法测量。在竞争性硼酸存在下的对照实验显示没有交叉副产物,并证实了硼酸酯/硫代半碳酰胺共轭物的稳定性和可逆性。结合物的形成不受诸如果糖,葡萄糖和儿茶酚等生物二醇的存在的影响,并且硫代氨基脲官能化的酚醛树脂对在蛋白质结合的乙醛基单元上发现的那种乙醛亲电子试剂是惰性的。使用SNAP-tag方法将硼酸试剂安装到HEK293T细胞中Beta-2肾上腺素受体的胞外域上,然后与最佳硫代氨基脲一起孵育,证明了该系统在活细胞细胞表面标记中的适用性。荧光素染料标记的功能化的壬二醇试剂。
更新日期:2017-10-03
中文翻译:

协同“点击”硼酸/硫代氨基脲系统在活细胞中快速和不可逆的生物正交偶联
对于使用无毒试剂的快速,高产量和选择性的生物正交“点击”反应,在活细胞中生物分子的缀合方面具有巨大的实用性,对此有很高的要求。尽管为此目的开发了许多单击反应,但许多单击反应都与缺点和局限有关,这些缺点和局限性证明了开发用于单标签或双标签应用程序的替代系统的合理性。最近的报道强调了硼酸酯形成的潜力,这是非生物硼酸和二醇之间的生物正交点击反应。硼酸酯的形成是一个快速的脱水过程。然而,它在水性介质中本质上是可逆的。我们设计和优化了基于两种双功能试剂,一种硫代氨基脲官能化的壬二醇和邻位双酚的协同系统。-乙酰基芳基硼酸。两种试剂在浓度高达50μM时对HEK293T细胞均具有化学稳定性和无毒性。所得的硼酸酯/硫代半碳zone加合物是一个中等大小的环,在中性缓冲液中,在低μM浓度下没有任何催化剂的情况下,快速且不可逆地形成,速率常数为9 M –1 s –1通过NMR光谱法测量。在竞争性硼酸存在下的对照实验显示没有交叉副产物,并证实了硼酸酯/硫代半碳酰胺共轭物的稳定性和可逆性。结合物的形成不受诸如果糖,葡萄糖和儿茶酚等生物二醇的存在的影响,并且硫代氨基脲官能化的酚醛树脂对在蛋白质结合的乙醛基单元上发现的那种乙醛亲电子试剂是惰性的。使用SNAP-tag方法将硼酸试剂安装到HEK293T细胞中Beta-2肾上腺素受体的胞外域上,然后与最佳硫代氨基脲一起孵育,证明了该系统在活细胞细胞表面标记中的适用性。荧光素染料标记的功能化的壬二醇试剂。































 京公网安备 11010802027423号
京公网安备 11010802027423号