当前位置:
X-MOL 学术
›
Inorg. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Redox-Active Bis(phenolate) N-Heterocyclic Carbene [OCO] Pincer Ligands Support Cobalt Electron Transfer Series Spanning Four Oxidation States
Inorganic Chemistry ( IF 4.3 ) Pub Date : 2017-10-02 00:00:00 , DOI: 10.1021/acs.inorgchem.7b01906 Caleb F. Harris 1 , Michael B. Bayless 1 , Nicolaas P. van Leest 2 , Quinton J. Bruch 1 , Brooke N. Livesay 3 , John Bacsa 1, 4 , Kenneth I. Hardcastle 4 , Matthew P. Shores 3 , Bas de Bruin 2 , Jake D. Soper 1
Inorganic Chemistry ( IF 4.3 ) Pub Date : 2017-10-02 00:00:00 , DOI: 10.1021/acs.inorgchem.7b01906 Caleb F. Harris 1 , Michael B. Bayless 1 , Nicolaas P. van Leest 2 , Quinton J. Bruch 1 , Brooke N. Livesay 3 , John Bacsa 1, 4 , Kenneth I. Hardcastle 4 , Matthew P. Shores 3 , Bas de Bruin 2 , Jake D. Soper 1
Affiliation
|
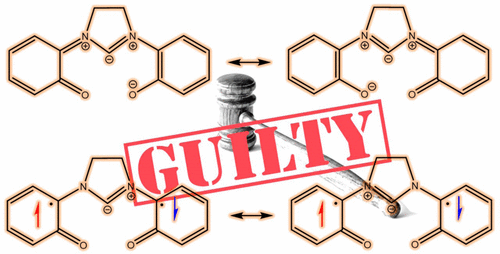
A new family of low-coordinate Co complexes supported by three redox-noninnocent tridentate [OCO] pincer-type bis(phenolate) N-heterocyclic carbene (NHC) ligands are described. Combined experimental and computational data suggest that the charge-neutral four-coordinate complexes are best formulated as Co(II) centers bound to closed-shell [OCO]2– dianions, of the general formula [(OCO)CoIIL] (where L is a solvent-derived MeCN or THF). Cyclic voltammograms of the [(OCO)CoIIL] complexes reveal three oxidations accessible at potentials below 1.2 V vs Fc+/Fc, corresponding to generation of formally Co(V) species, but the true physical/spectroscopic oxidation states are much lower. Chemical oxidations afford the mono- and dications of the imidazoline NHC-derived complex, which were examined by computational and magnetic and spectroscopic methods, including single-crystal X-ray diffraction. The metal and ligand oxidation states of the monocationic complex are ambiguous; data are consistent with formulation as either [(SOCO)CoIII(THF)2]+ containing a closed-shell [SOCO]2– diphenolate ligand bound to a S = 1 Co(III) center, or [(SOCO•)CoII(THF)2]+ with a low-spin Co(II) ion ferromagnetically coupled to monoanionic [SOCO•]− containing a single unpaired electron distributed across the [OCO] framework. The dication is best described as [(SOCO0)CoII(THF)3]2+, with a single unpaired electron localized on the d7 Co(II) center and a doubly oxidized, charge-neutral, closed-shell SOCO0 ligand. The combined data provide for the first time unequivocal and structural evidence for [OCO] ligand redox activity. Notably, varying the degree of unsaturation in the NHC backbone shifts the ligand-based oxidation potentials by up to 400 mV. The possible chemical origins of this unexpected shift, along with the potential utility of the [OCO] pincer ligands for base-metal-mediated organometallic coupling catalysis, are discussed.
中文翻译:

氧化还原活性双酚盐N-杂环碳烯[OCO]钳位配体支持跨越四个氧化态的钴电子转移系列
描述了由三个氧化还原-非纯三齿[OCO]钳型双(酚盐)N-杂环卡宾(NHC)配体支持的低配位Co配合物的新家族。结合实验和计算数据表明,最好将电荷中性的四配位配合物配制成与闭壳[OCO] 2-二价键结合的Co(II)中心,通式为[(OCO)Co II L](其中L是溶剂衍生的MeCN或THF。[(OCO)Co II L]配合物的循环伏安图显示,在相对于Fc +的电位低于1.2 V的电势下,可进行三种氧化/ Fc,对应于形式上的Co(V)物种的生成,但真正的物理/光谱氧化态要低得多。化学氧化作用提供了咪唑啉NHC衍生的配合物的单价和二价,可通过计算,磁性和光谱学方法(包括单晶X射线衍射)进行检查。单阳离子络合物的金属和配体氧化态是不明确的。数据与配方一致,因为[(S OCO)Co III(THF)2 ] +包含与S = 1 Co(III)中心键合的闭壳[ S OCO] 2-二酚盐配体或[[ S OCO •)Co II(THF)2 ] +具有低旋转的Co(II)离子铁磁耦合至单阴离子[ S OCO • ] -,该单阴离子[ S OCO • ] -包含分布在[OCO]骨架上的单个不成对电子。该指示最好描述为[(S OCO 0)Co II(THF)3 ] 2+,其中单个未配对电子位于d 7 Co(II)中心,并且具有双氧化性,电荷中性,闭壳S OCO 0配体。结合的数据首次为[OCO]配体氧化还原活性提供了明确和结构性的证据。值得注意的是,改变NHC主链中的不饱和度会使基于配体的氧化电势偏移多达400 mV。讨论了这种意外转变的可能化学起源,以及[OCO]钳形配体在贱金属介导的有机金属偶联催化中的潜在用途。
更新日期:2017-10-02
中文翻译:

氧化还原活性双酚盐N-杂环碳烯[OCO]钳位配体支持跨越四个氧化态的钴电子转移系列
描述了由三个氧化还原-非纯三齿[OCO]钳型双(酚盐)N-杂环卡宾(NHC)配体支持的低配位Co配合物的新家族。结合实验和计算数据表明,最好将电荷中性的四配位配合物配制成与闭壳[OCO] 2-二价键结合的Co(II)中心,通式为[(OCO)Co II L](其中L是溶剂衍生的MeCN或THF。[(OCO)Co II L]配合物的循环伏安图显示,在相对于Fc +的电位低于1.2 V的电势下,可进行三种氧化/ Fc,对应于形式上的Co(V)物种的生成,但真正的物理/光谱氧化态要低得多。化学氧化作用提供了咪唑啉NHC衍生的配合物的单价和二价,可通过计算,磁性和光谱学方法(包括单晶X射线衍射)进行检查。单阳离子络合物的金属和配体氧化态是不明确的。数据与配方一致,因为[(S OCO)Co III(THF)2 ] +包含与S = 1 Co(III)中心键合的闭壳[ S OCO] 2-二酚盐配体或[[ S OCO •)Co II(THF)2 ] +具有低旋转的Co(II)离子铁磁耦合至单阴离子[ S OCO • ] -,该单阴离子[ S OCO • ] -包含分布在[OCO]骨架上的单个不成对电子。该指示最好描述为[(S OCO 0)Co II(THF)3 ] 2+,其中单个未配对电子位于d 7 Co(II)中心,并且具有双氧化性,电荷中性,闭壳S OCO 0配体。结合的数据首次为[OCO]配体氧化还原活性提供了明确和结构性的证据。值得注意的是,改变NHC主链中的不饱和度会使基于配体的氧化电势偏移多达400 mV。讨论了这种意外转变的可能化学起源,以及[OCO]钳形配体在贱金属介导的有机金属偶联催化中的潜在用途。





















































 京公网安备 11010802027423号
京公网安备 11010802027423号