Journal of Catalysis ( IF 6.5 ) Pub Date : 2017-09-30 , DOI: 10.1016/j.jcat.2017.08.029 Hung-Chi Wu , Tse-Ching Chen , Yan-Chu Chen , Jyh-Fu Lee , Ching-Shiun Chen
|
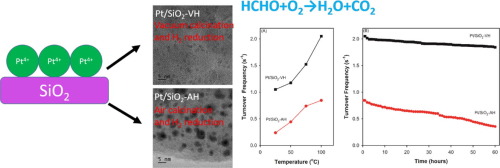
In this work, thermal treatment of 0.2 wt.% Pt4+ deposited on SiO2 through vacuum calcination and H2 reduction is used to synthesize Pt particles less than 1 nm in diameter that are uniformly dispersed on the SiO2. Traditional treatments involving air calcination/H2 reduction on the same 0.2 wt.% Pt4+/SiO2 result in the formation of inhomogeneous Pt particles with an average size of ∼8.4 nm. The tiny Pt particles have an improved reaction rate at ambient temperature and better catalytic stability for formaldehyde (HCHO) oxidation. The formation mechanism of tiny Pt particles and the reaction mechanism of HCHO oxidation were investigated. The CO decomposition product from HCHO could be the essential intermediate in the course of HCHO oxidation, and the oxidative reaction pathway should be HCHO → CO → CO2. Adsorbed HCHO may bond to the Pt surface in the η2 configuration and form CO through a decomposition process.
中文翻译:

二氧化硅负载的Pt催化剂上的甲醛氧化:热预处理对颗粒形成和氧化机理的影响
在该工作中,通过真空煅烧和H 2还原对SiO 2上沉积的0.2wt。%的Pt 4+进行热处理,以合成均匀分散在SiO 2上的直径小于1nm的Pt颗粒。传统处理包括在相同的0.2 wt。%Pt 4+ / SiO 2上进行空气煅烧/ H 2还原导致形成平均粒径约8.4 nm的不均匀Pt颗粒。微小的Pt颗粒在环境温度下具有提高的反应速率,并且对甲醛(HCHO)的氧化具有更好的催化稳定性。研究了Pt微粒的形成机理和HCHO氧化反应的机理。HCHO的CO分解产物可能是HCHO氧化过程中必不可少的中间体,其氧化反应途径应为HCHO→CO→CO 2。吸附的HCHO可以结合到Pt表面在η 2通过分解过程的配置和形式CO。





















































 京公网安备 11010802027423号
京公网安备 11010802027423号