当前位置:
X-MOL 学术
›
Anal. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Tool for Rapid Analysis of Glycopeptide by Permethylation via One-Pot Site Mapping and Glycan Analysis
Analytical Chemistry ( IF 6.7 ) Pub Date : 2017-10-02 00:00:00 , DOI: 10.1021/acs.analchem.7b01730 Asif Shajahan 1 , Nitin T. Supekar 1 , Christian Heiss 1 , Mayumi Ishihara 1 , Parastoo Azadi 1
Analytical Chemistry ( IF 6.7 ) Pub Date : 2017-10-02 00:00:00 , DOI: 10.1021/acs.analchem.7b01730 Asif Shajahan 1 , Nitin T. Supekar 1 , Christian Heiss 1 , Mayumi Ishihara 1 , Parastoo Azadi 1
Affiliation
|
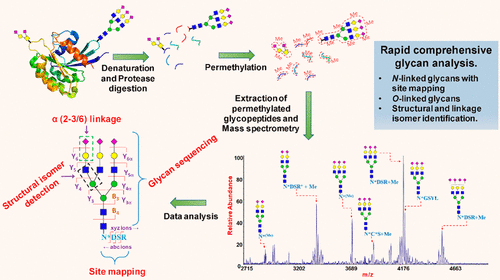
To overcome the challenges in the analysis of protein glycosylation, we have developed a comprehensive and universal tool through permethylation of glycopeptides and their tandem mass spectrometric analysis. This method has the potential to simplify glycoprotein analysis by integrating glycan sequencing and glycopeptide analysis in a single experiment. Moreover, glycans with unique glycosidic linkages, particularly from prokaryotes, which are resistant to enzymatic or chemical release, could also be detected and analyzed by this methodology. Here we present a strategy for the permethylation of intact glycopeptides, obtained via controlled protease digest, and their characterization by using advanced mass spectrometry. We used bovine RNase B, human transferrin, and bovine fetuin as models to demonstrate the feasibility of the method. Remarkably, the glycan patterns, glycosylation site, and their occupancy by N-glycans are all detected and identified in a single experimental procedure. Acquisition on a high resolution tandem-MSn system with fragmentation methodologies such as high-energy collision dissociation (HCD) and collision induced dissociation (CID), provided the complete sequence of the glycan structures attached to the peptides. The behavior of 20 natural amino acids under the basic permethylation conditions was probed by permethylating a library of short synthetic peptides. Our studies indicate that the permethylation imparts simple, limited, and predictable chemical transformations on peptides and do not interfere with the interpretation of MS/MS data. In addition to this, permethylated O-glycans in unreduced form (released by β elimination) were also detected, allowing us to profile O-linked glycan structures simultaneously.
中文翻译:

通过一锅位点定位和聚糖分析通过全甲基化快速分析糖肽的工具
为了克服蛋白质糖基化分析中的挑战,我们通过糖肽的全甲基化及其串联质谱分析开发了一种全面而通用的工具。通过在单个实验中整合聚糖测序和糖肽分析,该方法具有简化糖蛋白分析的潜力。此外,还可以通过这种方法检测和分析具有独特糖苷键的聚糖,特别是来自原核生物的,对酶促或化学释放具有抗性的聚糖。在这里,我们提出了一种通过控制蛋白酶消化获得完整糖肽的全甲基化的策略,以及通过使用先进的质谱技术对其进行表征的方法。我们使用牛核糖核酸酶B,人转铁蛋白和牛胎球蛋白作为模型来证明该方法的可行性。值得注意的是 聚糖的模式,糖基化位点及其在N-聚糖中的占有率均在单个实验过程中进行了检测和鉴定。高分辨率串联MS采集Ñ系统与碎片的方法,如高能碰撞解离(HCD)和碰撞诱导解离(CID),提供连接到所述肽聚糖结构的完整序列。通过短合成肽库的全甲基化,可以探查20种天然氨基酸在基本全甲基化条件下的行为。我们的研究表明,全甲基化作用使肽具有简单,有限和可预测的化学转化,并且不会干扰MS / MS数据的解释。除此之外,还检测到未还原形式的全甲基化O-聚糖(通过β消除释放),使我们能够同时分析O-连接的聚糖结构。
更新日期:2017-10-02
中文翻译:

通过一锅位点定位和聚糖分析通过全甲基化快速分析糖肽的工具
为了克服蛋白质糖基化分析中的挑战,我们通过糖肽的全甲基化及其串联质谱分析开发了一种全面而通用的工具。通过在单个实验中整合聚糖测序和糖肽分析,该方法具有简化糖蛋白分析的潜力。此外,还可以通过这种方法检测和分析具有独特糖苷键的聚糖,特别是来自原核生物的,对酶促或化学释放具有抗性的聚糖。在这里,我们提出了一种通过控制蛋白酶消化获得完整糖肽的全甲基化的策略,以及通过使用先进的质谱技术对其进行表征的方法。我们使用牛核糖核酸酶B,人转铁蛋白和牛胎球蛋白作为模型来证明该方法的可行性。值得注意的是 聚糖的模式,糖基化位点及其在N-聚糖中的占有率均在单个实验过程中进行了检测和鉴定。高分辨率串联MS采集Ñ系统与碎片的方法,如高能碰撞解离(HCD)和碰撞诱导解离(CID),提供连接到所述肽聚糖结构的完整序列。通过短合成肽库的全甲基化,可以探查20种天然氨基酸在基本全甲基化条件下的行为。我们的研究表明,全甲基化作用使肽具有简单,有限和可预测的化学转化,并且不会干扰MS / MS数据的解释。除此之外,还检测到未还原形式的全甲基化O-聚糖(通过β消除释放),使我们能够同时分析O-连接的聚糖结构。
















































 京公网安备 11010802027423号
京公网安备 11010802027423号