当前位置:
X-MOL 学术
›
J. Chem. Phys.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Seniority number description of potential energy surfaces: Symmetric dissociation of water, N2, C2, and Be2
The Journal of Chemical Physics ( IF 3.1 ) Pub Date : 2015-09-03 13:26:36 , DOI: 10.1063/1.4929904 Laimutis Bytautas 1 , Gustavo E. Scuseria 2, 3 , Klaus Ruedenberg 4
The Journal of Chemical Physics ( IF 3.1 ) Pub Date : 2015-09-03 13:26:36 , DOI: 10.1063/1.4929904 Laimutis Bytautas 1 , Gustavo E. Scuseria 2, 3 , Klaus Ruedenberg 4
Affiliation
|
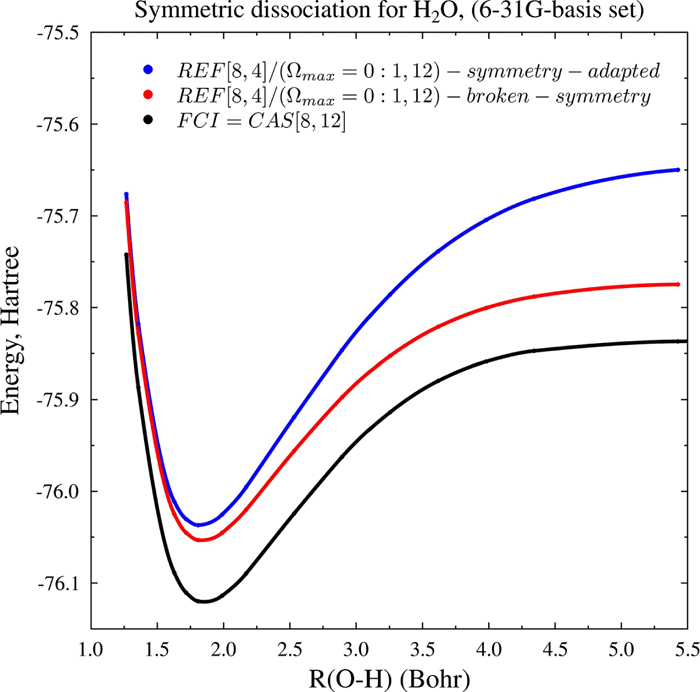
The present study further explores the concept of the seniority number (Ω) by examining different configuration interaction (CI) truncation strategies in generating compact wave functions in a systematic way. While the role of Ω in addressing static (strong) correlation problem has been addressed in numerous previous studies, the usefulness of seniority number in describing weak (dynamic) correlation has not been investigated in a systematic way. Thus, the overall objective in the present work is to investigate the role of Ω in addressing also dynamic electron correlation in addition to the static correlation. Two systematic CI truncation strategies are compared beyond minimal basis sets and full valence active spaces. One approach is based on the seniority number (defined as the total number of singly occupied orbitals in a determinant) and another is based on an excitation-level limitation. In addition, molecular orbitals are energy-optimized using multiconfigurational-self-consistent-field procedure for all these wave functions. The test cases include the symmetric dissociation of water (6-31G), N2 (6-31G), C2 (6-31G), and Be2 (cc-pVTZ). We find that the potential energy profile for H2O dissociation can be reasonably well described using only the Ω = 0 sector of the CI wave function. For the Be2 case, we show that the full CI potential energy curve (cc-pVTZ) is almost exactly reproduced using either Ω-based (including configurations having up to Ω = 2 in the virtual-orbital-space) or excitation-based (up to single-plus-double-substitutions) selection methods, both out of a full-valence-reference function. Finally, in dissociation cases of N2 and C2, we shall also consider novel hybrid wave functions obtained by a union of a set of CI configurations representing the full valence space and a set of CI configurations where seniority-number restriction is imposed for a complete set (full-valence-space and virtual) of correlated molecular orbitals, simultaneously. We discuss the usefulness of the seniority number concept in addressing both static and dynamic electron correlation problems along dissociation paths.
中文翻译:

势能面的资历数字描述:水,N2,C2和Be2的对称解离
本研究通过检查系统地生成紧凑波函数中的不同配置相互作用(CI)截断策略,进一步探索了资历数(Ω)的概念。尽管先前的许多研究都已经论证了Ω在解决静态(强)相关性问题中的作用,但尚未系统地研究过资历数字在描述弱(动态)相关性方面的有用性。因此,本工作的总体目标是研究Ω在解决静态相关性以及动态电子相关性方面的作用。两个系统的CI截断策略相比,超出了最小基集和全价活动空间。一种方法是基于优先级数(定义为行列式中单个占据的轨道总数),另一种方法是基于激励级别限制。此外,对于所有这些波函数,都使用多配置自洽场过程对分子轨道进行了能量优化。测试案例包括水(6-31G),N 2(6-31G),C 2(6-31G)和Be 2(cc-pVTZ)的对称解离。我们发现,仅使用CI波函数的Ω= 0扇区,就可以合理地很好地描述H 2 O离解的势能分布。对于Be 2在这种情况下,我们显示了完整的CI势能曲线(cc-pVTZ)几乎可以使用基于Ω(包括在虚拟轨道空间中具有Ω= 2的配置)或基于激励(高达单个-plus-double-substitutions)选择方法,这两种方法均不具有全价参考功能。最后,在N 2和C 2离解的情况下,我们还将考虑通过并集获得的新型混合波函数代表全价空间的一组CI构型和一组CI配置的组,其中对相关分子轨道的完整集(全价空间和虚拟)同时进行了资历数限制。我们讨论了资历数字概念在解决沿解离路径的静态和动态电子相关性问题中的有用性。
更新日期:2015-09-04
中文翻译:

势能面的资历数字描述:水,N2,C2和Be2的对称解离
本研究通过检查系统地生成紧凑波函数中的不同配置相互作用(CI)截断策略,进一步探索了资历数(Ω)的概念。尽管先前的许多研究都已经论证了Ω在解决静态(强)相关性问题中的作用,但尚未系统地研究过资历数字在描述弱(动态)相关性方面的有用性。因此,本工作的总体目标是研究Ω在解决静态相关性以及动态电子相关性方面的作用。两个系统的CI截断策略相比,超出了最小基集和全价活动空间。一种方法是基于优先级数(定义为行列式中单个占据的轨道总数),另一种方法是基于激励级别限制。此外,对于所有这些波函数,都使用多配置自洽场过程对分子轨道进行了能量优化。测试案例包括水(6-31G),N 2(6-31G),C 2(6-31G)和Be 2(cc-pVTZ)的对称解离。我们发现,仅使用CI波函数的Ω= 0扇区,就可以合理地很好地描述H 2 O离解的势能分布。对于Be 2在这种情况下,我们显示了完整的CI势能曲线(cc-pVTZ)几乎可以使用基于Ω(包括在虚拟轨道空间中具有Ω= 2的配置)或基于激励(高达单个-plus-double-substitutions)选择方法,这两种方法均不具有全价参考功能。最后,在N 2和C 2离解的情况下,我们还将考虑通过并集获得的新型混合波函数代表全价空间的一组CI构型和一组CI配置的组,其中对相关分子轨道的完整集(全价空间和虚拟)同时进行了资历数限制。我们讨论了资历数字概念在解决沿解离路径的静态和动态电子相关性问题中的有用性。





















































 京公网安备 11010802027423号
京公网安备 11010802027423号