当前位置:
X-MOL 学术
›
ACS Catal.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Mild and Efficient Ni-Catalyzed Biaryl Synthesis with Polyfluoroaryl Magnesium Species: Verification of the Arrest State, Uncovering the Hidden Competitive Second Transmetalation and Ligand-Accelerated Highly Selective Monoarylation
ACS Catalysis ( IF 11.3 ) Pub Date : 2017-09-29 00:00:00 , DOI: 10.1021/acscatal.7b02618 Junya Wang 1 , Ge Meng 1 , Kun Xie 1 , Liting Li 1 , Huaming Sun 1 , Zhiyan Huang 1
ACS Catalysis ( IF 11.3 ) Pub Date : 2017-09-29 00:00:00 , DOI: 10.1021/acscatal.7b02618 Junya Wang 1 , Ge Meng 1 , Kun Xie 1 , Liting Li 1 , Huaming Sun 1 , Zhiyan Huang 1
Affiliation
|
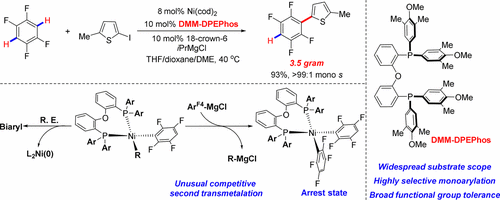
Employing a nickel catalyst and electron-deficient polyfluoroaryl magnesium species, a highly selective monoarylation of polyfluoroarenes containing multiple identical coupling sites has been achieved for the first time, which represents a long-standing problem due to the competitive reactivity between the desired products and the starting polyfluoroarenes. Because of the negative fluorine effect, a surprisingly stable cis [Ni(ArF4)2(DPEPhos)] species 4 (ArF4 = 2,3,5,6-tetrafluorophenyl) confirmed by X-ray crystallography is isolated, which acts as catalyst arrest state as proven by a thermal decomposition test. Further retro-transmetalation experiments uncover a hidden secondary transmetalation between ArF4-Ni-Ph and excess ArF4-MgCl that competes with the desired but reluctant reductive elimination at the high-valent nickel center. Accordingly, through the cooperation of newly developed DMM-DPEPhos, and a dioxane-mediated Schlenk equilibrium with Grignard reagent, the formation of the corresponding arrest state is remarkably inhibited. An excellent coupling efficiency and an excellent monoarylation selectivity are therefore generally accomplished with a widespread electrophile scope and good functional group tolerance under mild conditions. Importantly, our novel method shows great power in the gram-scale synthesis of thienyl-2,3,5,6-tetrafluorophenyl units that represent key components in materials science.
中文翻译:

多氟芳基镁物种的温和高效镍催化联芳基合成:逮捕状态的验证,发现隐藏的竞争性第二重金属化和配体加速的高选择性单芳基化
利用镍催化剂和缺电子的多氟芳基镁物质,首次实现了具有多个相同偶联位点的多氟芳烃的高选择性单芳基化,由于所需产物与起始原料之间的竞争反应性,这是一个长期存在的问题。多氟芳烃。由于负氟效应,分离出了通过X射线晶体学证实的出乎意料的稳定的顺式[Ni(Ar F4)2(DPEPhos)]种类4(Ar F4 = 2,3,5,6-四氟苯基),其作用与通过热分解试验证明催化剂的阻滞状态。进一步的反重金属化实验发现了Ar之间隐藏的二次重金属化F4 -Ni-Ph和过量的Ar F4- MgCl在高价镍中心与所需的但不希望发生的还原性消除竞争。因此,通过新开发的DMM-DPEPhos的协同作用,以及与格氏试剂的二恶烷介导的Schlenk平衡,显着抑制了相应的阻滞状态的形成。因此,一般的亲和力范围和在温和条件下良好的官能团耐受性通常可实现出色的偶联效率和出色的单芳基选择性。重要的是,我们的新方法在噻吩-2,3,5,6-四氟苯基单元的克级合成中显示出强大的能力,这些单元代表了材料科学中的关键成分。
更新日期:2017-09-29
中文翻译:

多氟芳基镁物种的温和高效镍催化联芳基合成:逮捕状态的验证,发现隐藏的竞争性第二重金属化和配体加速的高选择性单芳基化
利用镍催化剂和缺电子的多氟芳基镁物质,首次实现了具有多个相同偶联位点的多氟芳烃的高选择性单芳基化,由于所需产物与起始原料之间的竞争反应性,这是一个长期存在的问题。多氟芳烃。由于负氟效应,分离出了通过X射线晶体学证实的出乎意料的稳定的顺式[Ni(Ar F4)2(DPEPhos)]种类4(Ar F4 = 2,3,5,6-四氟苯基),其作用与通过热分解试验证明催化剂的阻滞状态。进一步的反重金属化实验发现了Ar之间隐藏的二次重金属化F4 -Ni-Ph和过量的Ar F4- MgCl在高价镍中心与所需的但不希望发生的还原性消除竞争。因此,通过新开发的DMM-DPEPhos的协同作用,以及与格氏试剂的二恶烷介导的Schlenk平衡,显着抑制了相应的阻滞状态的形成。因此,一般的亲和力范围和在温和条件下良好的官能团耐受性通常可实现出色的偶联效率和出色的单芳基选择性。重要的是,我们的新方法在噻吩-2,3,5,6-四氟苯基单元的克级合成中显示出强大的能力,这些单元代表了材料科学中的关键成分。





















































 京公网安备 11010802027423号
京公网安备 11010802027423号