当前位置:
X-MOL 学术
›
Tetrahedron Lett.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Stereoselective synthesis of two potential metabolites of cis-metconazole
Tetrahedron Letters ( IF 1.5 ) Pub Date : 2017-09-30 , DOI: 10.1016/j.tetlet.2017.09.087 Marcin Budny , Joanna Włodarczyk , Tadeusz Muzioł , Mariusz Jan Bosiak , Andrzej Wolan
中文翻译:

立体选择性合成顺式甲康唑的两种潜在代谢物
更新日期:2017-09-30
Tetrahedron Letters ( IF 1.5 ) Pub Date : 2017-09-30 , DOI: 10.1016/j.tetlet.2017.09.087 Marcin Budny , Joanna Włodarczyk , Tadeusz Muzioł , Mariusz Jan Bosiak , Andrzej Wolan
|
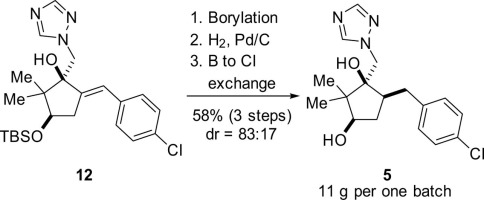
The stereoselective synthesis of compounds 5 and 6, potential hydroxylated metabolites of the agricultural fungicide cis-metconazole, is reported. In a key step of the initially surveyed synthetic route, hydrodechlorination of 12 was competitive with hydrogenation of the trisubstituted olefin. Application of a Miyaura borylation/hydrogenation/boron-to-halogen exchange reaction sequence solved the chemoselectivity issue.
中文翻译:

立体选择性合成顺式甲康唑的两种潜在代谢物
报道了化合物5和6的立体选择性合成,所述化合物5和6是农业杀真菌剂顺式-甲康唑的潜在羟基化代谢产物。在最初研究的合成路线的关键步骤中,将12的加氢脱氯与三取代的烯烃的加氢竞争。宫浦硼化/氢化/硼-卤素交换反应序列的应用解决了化学选择性问题。































 京公网安备 11010802027423号
京公网安备 11010802027423号