当前位置:
X-MOL 学术
›
Tetrahedron Lett.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
An efficient synthesis of the 1,6-dioxaspiro[4.4]nonan-2-one motif of the immunosuppressive triterpenoid Phainanoid F
Tetrahedron Letters ( IF 1.5 ) Pub Date : 2017-09-30 , DOI: 10.1016/j.tetlet.2017.09.091 Chen-Lu Zhang , Fa-Jun Nan
中文翻译:

有效合成免疫抑制三萜类化合物Phainanoid F的1,6-dioxaspiro [4.4] nonan-2-one主题
更新日期:2017-09-30
Tetrahedron Letters ( IF 1.5 ) Pub Date : 2017-09-30 , DOI: 10.1016/j.tetlet.2017.09.091 Chen-Lu Zhang , Fa-Jun Nan
|
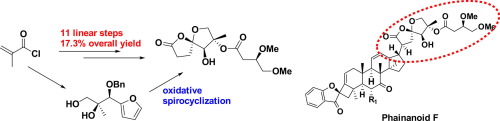
We describe an efficient synthesis of the 1,6-dioxaspiro[4.4]nonan-2-one motif of the immunosuppressive triterpenoid Phainanoid F and its C4 epimer. A furan oxidative spirocyclization for constructing the spiro center was used as the key step. Other important reactions involved Sharpless asymmetric dihydroxylation, Weinreb ketone synthesis and Yamaguchi esterfication. The synthesis was achieved in 11 linear steps with 17.3% overall yield.
中文翻译:

有效合成免疫抑制三萜类化合物Phainanoid F的1,6-dioxaspiro [4.4] nonan-2-one主题
我们描述了一种免疫抑制性三萜类化合物Phainanoid F及其C4差向异构体的1,6-dioxaspiro [4.4] nonan-2-one主题的有效合成。用于构建螺中心的呋喃氧化螺环化是关键步骤。其他重要反应包括Sharpless不对称二羟基化反应,Weinreb酮合成和Yamaguchi酯化反应。该合成以11个线性步骤完成,总产率为17.3%。






























 京公网安备 11010802027423号
京公网安备 11010802027423号