当前位置:
X-MOL 学术
›
Nano Energy
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Lithium malonatoborate additives enabled stable cycling of 5 V lithium metal and lithium ion batteries☆
Nano Energy ( IF 16.8 ) Pub Date : 2017-08-01 , DOI: 10.1016/j.nanoen.2017.07.051 Yunchao Li , Gabriel M. Veith , Katie L. Browning , Jihua Chen , Dale K. Hensley , Mariappan Parans Paranthaman , Sheng Dai , Xiao-Guang Sun
Nano Energy ( IF 16.8 ) Pub Date : 2017-08-01 , DOI: 10.1016/j.nanoen.2017.07.051 Yunchao Li , Gabriel M. Veith , Katie L. Browning , Jihua Chen , Dale K. Hensley , Mariappan Parans Paranthaman , Sheng Dai , Xiao-Guang Sun
|
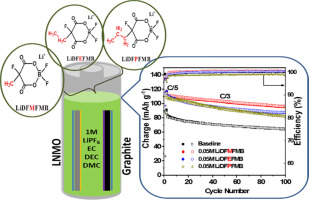
A series of lithium difluoro-2-fluoro-2-alkyl-malonatoborate salts have been used as additives in conventional 1.0 M LiPF6/ethylene carbonate (EC)-dimethyl carbonate (DMC)-diethyl carbonate (DEC) (1-1-1, by v) electrolyte for high voltage LiNi0.5Mn1.5O4 (LNMO) based lithium metal and lithium ion batteries. Cyclic voltammograms (CVs) reveal that the electrolytes with additives can significantly suppress the co-intercalation of solvents into the graphene layers during the first cycle due to their sacrificial reductions on the surface of the graphite electrode above 1.0 V vs Li/Li+. In addition, CVs reveal that the electrolyte without additive suffers from extensive electrolyte oxidation on the surface of the LNMO electrode during the first cycle, resulting in the biggest increase of the total cell impedance. Furthermore, electrochemical floating test shows less oxidation current in the electrolytes with additives at voltages above 5.0 V, proving good passivation by the additives. More importantly, the presence of additives can effectively increase the first cycle coulombic efficiencies and cycling stability in the LNMO based lithium metal and lithium ion batteries. Scanning electron microscope (SEM) and X-ray photoelectron spectroscopy (XPS) show that with additives compact solid electrolyte interphase (SEI) and thinner passivation layer are formed on the surfaces of the graphite and LNMO electrode, respectively. Finally, these salt additives can better protect the current collector from corrosion, further confirming their effectiveness in conventional electrolytes for high-voltage lithium metal and lithium ion batteries.
中文翻译:

丙硼酸锂添加剂可使5 V锂金属和锂离子电池稳定循环☆
在传统的1.0 M LiPF 6 /碳酸亚乙酯(EC)-碳酸二甲酯(DMC)-碳酸二乙酯(DEC-)(1-1-参照图1,通过v)用于高压LiNi 0.5 Mn 1.5 O 4(LNMO)的锂金属和锂离子电池的电解质。循环伏安图(CVs)显示,具有添加剂的电解质可在第一个循环中显着抑制溶剂共嵌入石墨烯层中,这是由于它们在石墨电极表面上的牺牲性降低超过1.0 V vs Li / Li + 。此外,CVs显示,在第一个循环中,不含添加剂的电解质会在LNMO电极的表面遭受广泛的电解质氧化,从而导致总电池阻抗的最大增加。此外,电化学浮动测试显示,在电压高于5.0的条件下,具有添加剂的电解质中的氧化电流较小 V,证明添加剂具有良好的钝化作用。更重要的是,添加剂的存在可以有效地提高基于LNMO的锂金属和锂离子电池的第一循环库仑效率和循环稳定性。扫描电子显微镜(SEM)和X射线光电子能谱(XPS)表明,使用添加剂,分别在石墨和LNMO电极的表面上形成了致密的固体电解质相(SEI)和更薄的钝化层。最后,这些盐添加剂可以更好地保护集电器免受腐蚀,进一步证实了它们在用于高压锂金属和锂离子电池的常规电解质中的有效性。
更新日期:2017-09-29
中文翻译:

丙硼酸锂添加剂可使5 V锂金属和锂离子电池稳定循环☆
在传统的1.0 M LiPF 6 /碳酸亚乙酯(EC)-碳酸二甲酯(DMC)-碳酸二乙酯(DEC-)(1-1-参照图1,通过v)用于高压LiNi 0.5 Mn 1.5 O 4(LNMO)的锂金属和锂离子电池的电解质。循环伏安图(CVs)显示,具有添加剂的电解质可在第一个循环中显着抑制溶剂共嵌入石墨烯层中,这是由于它们在石墨电极表面上的牺牲性降低超过1.0 V vs Li / Li + 。此外,CVs显示,在第一个循环中,不含添加剂的电解质会在LNMO电极的表面遭受广泛的电解质氧化,从而导致总电池阻抗的最大增加。此外,电化学浮动测试显示,在电压高于5.0的条件下,具有添加剂的电解质中的氧化电流较小 V,证明添加剂具有良好的钝化作用。更重要的是,添加剂的存在可以有效地提高基于LNMO的锂金属和锂离子电池的第一循环库仑效率和循环稳定性。扫描电子显微镜(SEM)和X射线光电子能谱(XPS)表明,使用添加剂,分别在石墨和LNMO电极的表面上形成了致密的固体电解质相(SEI)和更薄的钝化层。最后,这些盐添加剂可以更好地保护集电器免受腐蚀,进一步证实了它们在用于高压锂金属和锂离子电池的常规电解质中的有效性。


















































 京公网安备 11010802027423号
京公网安备 11010802027423号