Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Understanding the Formation of CaAl2Si2O8 in Melilite-Based Glass-Ceramics: Combined Diffraction and Spectroscopic Studies
ACS Omega ( IF 3.7 ) Pub Date : 2017-09-28 00:00:00 , DOI: 10.1021/acsomega.7b00598
Amarnath R. Allu 1, 2 , Sathravada Balaji 1 , Dilshat U. Tulyaganov 2, 3 , Glenn C. Mather 4 , Fabian Margit 5 , María J. Pascual 4 , Renée Siegel , Wolfgang Milius , Jürgen Senker , Dmitrii A. Agarkov 6 , Vladislav V. Kharton 6 , José M. F. Ferreira 2
ACS Omega ( IF 3.7 ) Pub Date : 2017-09-28 00:00:00 , DOI: 10.1021/acsomega.7b00598
Amarnath R. Allu 1, 2 , Sathravada Balaji 1 , Dilshat U. Tulyaganov 2, 3 , Glenn C. Mather 4 , Fabian Margit 5 , María J. Pascual 4 , Renée Siegel , Wolfgang Milius , Jürgen Senker , Dmitrii A. Agarkov 6 , Vladislav V. Kharton 6 , José M. F. Ferreira 2
Affiliation
|
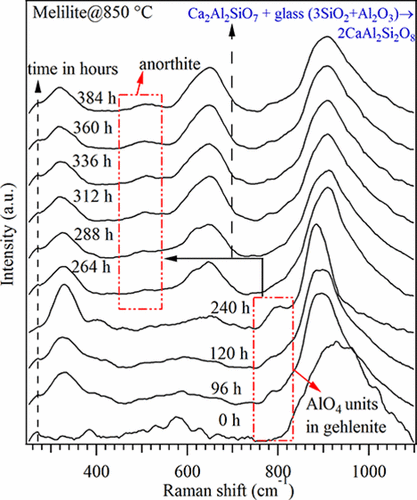
An assessment is undertaken for the formation of anorthite crystalline phase in a melilite-based glass composition (CMAS: 38.7CaO–9.7MgO–12.9Al2O3–38.7SiO2 mol %), used as a sealing material in solid oxide fuel cells, in view of the detrimental effect of anorthite on the sealing properties. Several advanced characterization techniques are employed to assess the material after prolonged heat treatment, including neutron powder diffraction (ND), X-ray powder diffraction (XRD), 29Si and 27Al magic-angle spinning nuclear magnetic resonance (MAS-NMR), and in situ Raman spectroscopy. ND, 29Si MAS-NMR, and 27Al MAS-NMR results revealed that both Si and Al adopt tetrahedral coordination and participate in the formation of the network structure. In situ XRD measurements for the CMAS glass demonstrate the thermal stability of the glass structure up to 850 °C. Further heat treatment up to 900 °C initiates the precipitation of melilite, a solid solution of akermanite/gehlenite crystalline phase. Qualitative XRD data for glass-ceramics (GCs) produced after heat treatment at 850 °C for 500 h revealed the presence of anorthite along with the melilite crystalline phase. Rietveld refinement of XRD data indicated a high fraction of glassy phase (∼67%) after the formation of crystalline phases. The 29Si MAS-NMR spectra for the CMAS-GC suggest the presence of structural units in the remaining glassy phase with a polymerization degree higher than dimer units, whereas the 27Al MAS-NMR spectra revealed that most Al3+ cations exhibit a 4-fold coordination. In situ Raman spectroscopy data indicate that the formation of anorthite crystalline phase initiated after 240 h of heat treatment at 850 °C owing to the interaction between the gehlenite crystals and the remaining glassy phase.
中文翻译:

理解基于沸石的玻璃陶瓷中CaAl 2 Si 2 O 8的形成:衍射和光谱研究的结合
进行了评估在基于陨石的玻璃成分(CMAS:38.7CaO–9.7MgO–12.9Al 2 O 3 –38.7SiO 2 mol%)中形成钙长石结晶相的研究,该固体氧化物燃料电池用作固体氧化物燃料电池的密封材料考虑到钙长石对密封性能的不利影响。长时间热处理后,采用了几种先进的表征技术来评估材料,包括中子粉末衍射(ND),X射线粉末衍射(XRD),29 Si和27 Al魔角旋转核磁共振(MAS-NMR),和原位拉曼光谱。ND,29 Si MAS-NMR和27Al MAS-NMR结果表明,Si和Al均采用四面体配位并参与网络结构的形成。CMAS玻璃的原位XRD测量表明,该玻璃结构在850°C的温度下具有热稳定性。最高至900°C的进一步热处理会引发镁铝石沉淀,这是一种钙钛矿/方钠石结晶相的固溶体。在850°C热处理500 h后生产的玻璃陶瓷(GC)的定性XRD数据表明,钙长石与镁红柱石晶相一起存在。XRD数据的Rietveld精炼表明,在形成结晶相后,玻璃相的比例较高(约67%)。在29CMAS-GC的Si MAS-NMR光谱表明,剩余玻璃相中存在结构单元,其聚合度高于二聚体,而27 Al MAS-NMR光谱表明,大多数Al 3+阳离子显示4倍协调。原位拉曼光谱数据表明,由于斜晶石晶体和其余玻璃态之间的相互作用,在850°C热处理240小时后,钙长石晶体相开始形成。
更新日期:2017-09-28
中文翻译:

理解基于沸石的玻璃陶瓷中CaAl 2 Si 2 O 8的形成:衍射和光谱研究的结合
进行了评估在基于陨石的玻璃成分(CMAS:38.7CaO–9.7MgO–12.9Al 2 O 3 –38.7SiO 2 mol%)中形成钙长石结晶相的研究,该固体氧化物燃料电池用作固体氧化物燃料电池的密封材料考虑到钙长石对密封性能的不利影响。长时间热处理后,采用了几种先进的表征技术来评估材料,包括中子粉末衍射(ND),X射线粉末衍射(XRD),29 Si和27 Al魔角旋转核磁共振(MAS-NMR),和原位拉曼光谱。ND,29 Si MAS-NMR和27Al MAS-NMR结果表明,Si和Al均采用四面体配位并参与网络结构的形成。CMAS玻璃的原位XRD测量表明,该玻璃结构在850°C的温度下具有热稳定性。最高至900°C的进一步热处理会引发镁铝石沉淀,这是一种钙钛矿/方钠石结晶相的固溶体。在850°C热处理500 h后生产的玻璃陶瓷(GC)的定性XRD数据表明,钙长石与镁红柱石晶相一起存在。XRD数据的Rietveld精炼表明,在形成结晶相后,玻璃相的比例较高(约67%)。在29CMAS-GC的Si MAS-NMR光谱表明,剩余玻璃相中存在结构单元,其聚合度高于二聚体,而27 Al MAS-NMR光谱表明,大多数Al 3+阳离子显示4倍协调。原位拉曼光谱数据表明,由于斜晶石晶体和其余玻璃态之间的相互作用,在850°C热处理240小时后,钙长石晶体相开始形成。

































 京公网安备 11010802027423号
京公网安备 11010802027423号