当前位置:
X-MOL 学术
›
J. Phys. Chem. C
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Electronic Structure of the [Cu3(μ-O)3]2+ Cluster in Mordenite Zeolite and Its Effects on the Methane to Methanol Oxidation
The Journal of Physical Chemistry C ( IF 3.3 ) Pub Date : 2017-09-28 00:00:00 , DOI: 10.1021/acs.jpcc.7b08714
Konstantinos D. Vogiatzis 1 , Guanna Li 2, 3 , Emiel J. M. Hensen 2, 4 , Laura Gagliardi 5 , Evgeny A. Pidko 2, 4, 6
The Journal of Physical Chemistry C ( IF 3.3 ) Pub Date : 2017-09-28 00:00:00 , DOI: 10.1021/acs.jpcc.7b08714
Konstantinos D. Vogiatzis 1 , Guanna Li 2, 3 , Emiel J. M. Hensen 2, 4 , Laura Gagliardi 5 , Evgeny A. Pidko 2, 4, 6
Affiliation
|
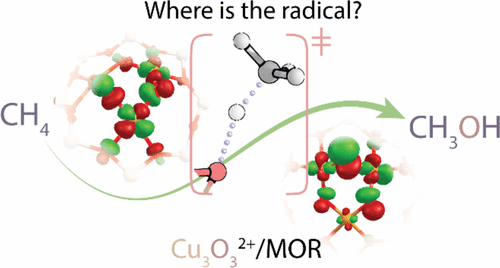
Identifying Cu-exchanged zeolites able to activate C–H bonds and selectively convert methane to methanol is a challenge in the field of biomimetic heterogeneous catalysis. Recent experiments point to the importance of trinuclear [Cu3(μ-O)3]2+ complexes inside the micropores of mordenite (MOR) zeolite for selective oxo-functionalization of methane. The electronic structures of these species, namely, the oxidation state of Cu ions and the reactive character of the oxygen centers, are not yet fully understood. In this study, we performed a detailed analysis of the electronic structure of the [Cu3(μ-O)3]2+ site using multiconfigurational wave-function-based methods and density functional theory. The calculations reveal that all Cu sites in the cluster are predominantly present in the Cu(II) formal oxidation state with a minor contribution from Cu(III), whereas two out of three oxygen anions possess a radical character. These electronic properties, along with the high accessibility of the out-of-plane oxygen center, make this oxygen the preferred site for the homolytic C–H activation of methane by [Cu3(μ-O)3]2+. These new insights aid in the construction of a theoretical framework for the design of novel catalysts for oxyfunctionalization of natural gas and suggest further spectroscopic examination.
中文翻译:

丝光沸石中[Cu 3(μ-O)3 ] 2+簇的电子结构及其对甲烷氧化甲醇氧化的影响
在仿生非均相催化领域,鉴定能够活化CH键并选择性将甲烷转化为甲醇的Cu交换沸石是一项挑战。最近的实验指出,丝光沸石(MOR)沸石微孔内部的三核[Cu 3(μ-O)3 ] 2+络合物对于甲烷的选择性羰基官能化非常重要。这些物质的电子结构,即Cu离子的氧化态和氧中心的反应性,尚未完全了解。在这项研究中,我们对[Cu 3(μ-O)3 ] 2+的电子结构进行了详细分析。站点使用基于多配置波函数的方法和密度泛函理论。计算表明,簇中的所有Cu位点都主要以Cu(II)形式氧化态存在,而Cu(III)的贡献很小,而三个氧负离子中有两个具有自由基特性。这些电子特性以及平面外氧中心的高度可及性使该氧成为[Cu 3(μ-O)3 ] 2+对甲烷进行均相C–H活化的优选位点。这些新见解有助于构建用于天然气氧官能化的新型催化剂设计的理论框架,并建议进一步的光谱学检查。
更新日期:2017-09-28
中文翻译:

丝光沸石中[Cu 3(μ-O)3 ] 2+簇的电子结构及其对甲烷氧化甲醇氧化的影响
在仿生非均相催化领域,鉴定能够活化CH键并选择性将甲烷转化为甲醇的Cu交换沸石是一项挑战。最近的实验指出,丝光沸石(MOR)沸石微孔内部的三核[Cu 3(μ-O)3 ] 2+络合物对于甲烷的选择性羰基官能化非常重要。这些物质的电子结构,即Cu离子的氧化态和氧中心的反应性,尚未完全了解。在这项研究中,我们对[Cu 3(μ-O)3 ] 2+的电子结构进行了详细分析。站点使用基于多配置波函数的方法和密度泛函理论。计算表明,簇中的所有Cu位点都主要以Cu(II)形式氧化态存在,而Cu(III)的贡献很小,而三个氧负离子中有两个具有自由基特性。这些电子特性以及平面外氧中心的高度可及性使该氧成为[Cu 3(μ-O)3 ] 2+对甲烷进行均相C–H活化的优选位点。这些新见解有助于构建用于天然气氧官能化的新型催化剂设计的理论框架,并建议进一步的光谱学检查。

































 京公网安备 11010802027423号
京公网安备 11010802027423号