Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Characterization of Repulsive Forces and Surface Deformation in Thin Micellar Films via AFM
Langmuir ( IF 3.7 ) Pub Date : 2017-09-26 00:00:00 , DOI: 10.1021/acs.langmuir.7b02508 Benjamin L. Micklavzina 1 , Marjorie L. Longo 2
Langmuir ( IF 3.7 ) Pub Date : 2017-09-26 00:00:00 , DOI: 10.1021/acs.langmuir.7b02508 Benjamin L. Micklavzina 1 , Marjorie L. Longo 2
Affiliation
|
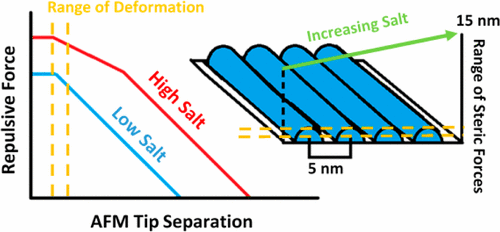
Here we examine how the force on an atomic force microscope (AFM) tip varies as it approaches micellar surfactant films, and use this information to discern the film’s surface structure and Young’s modulus. Rows of wormlike hemimicelles were created at a graphite interface using 10 mM sodium dodecyl sulfate (SDS). We found that the repulsive force on a silicon nitride tip as it approached the surface was exponential, with a decay length of 2.0 ± 0.1 nm. The addition of Na2SO4 was found to cause a change in this behavior, with a clear split into two exponential regions at concentrations above 1 mM. We also observed that the range of these forces increased with added salt from ∼15 nm in pure SDS to ∼20 nm at a Na2SO4 concentration of 1.34 mM. These forces were inconsistent with electrostatic repulsion, and were determined to be steric in nature. We show that the behavior at higher salt concentrations is consistent with the theory of polyelectrolyte brushes in the osmotic regime. From this, we hypothesize the presence of micellar brushes at the surface that behave similarly to adsorbed polymer chains. In addition, the Young’s modulus of the film was taken from data near the interface using Sneddon’s model, and found to be 80 ± 40 MPa. Similar experiments were performed with 10 mM dodecylamine hydrochloride (DAH) solutions in the presence of added magnesium chloride. The decay length for the pure DAH solution was found to be 2.6 ± 0.3 nm, and the addition of 1.34 mM of MgCl2 caused this to increase to 3.7 ± 0.3 nm. No decay length splitting was observed for DAH. We conclude that the behavior at the surface resembles that of an uncharged polymer brush, as the ionic and surface charge densities are much lower for DAH than for SDS.
中文翻译:

通过原子力显微镜表征胶束薄膜中的排斥力和表面变形
在这里,我们研究了原子力显微镜(AFM)尖端接近胶束表面活性剂膜时的力如何变化,并使用此信息来识别膜的表面结构和杨氏模量。使用10 mM十二烷基硫酸钠(SDS)在石墨界面上产生成排的蠕虫状半胶束。我们发现,氮化硅尖端接近表面时的排斥力呈指数级,衰减长度为2.0±0.1 nm。发现添加Na 2 SO 4会引起这种行为的改变,并且在浓度高于1 mM时清晰地分成两个指数区域。我们还观察到,这些作用力的范围随添加的盐从纯SDS中的〜15 nm增加到Na 2 SO 4下的〜20 nm而增加。浓度为1.34 mM。这些力与静电排斥力不一致,并且被确定为空间位阻。我们表明,在较高盐浓度下的行为与渗透体系中的聚电解质刷的理论是一致的。据此,我们假设表面上存在与吸附的聚合物链相似的胶束刷。另外,使用Sneddon模型从界面附近的数据获取膜的杨氏模量,发现为80±40MPa。在添加氯化镁的情况下,用10 mM十二烷基胺盐酸盐(DAH)溶液进行了类似的实验。发现纯DAH溶液的衰减长度为2.6±0.3 nm,并添加了1.34 mM的MgCl 2使它增加到3.7±0.3 nm。对于DAH,未观察到衰变长度分裂。我们得出的结论是,表面的行为类似于不带电的聚合物刷,因为DAH的离子和表面电荷密度远低于SDS。
更新日期:2017-09-26
中文翻译:

通过原子力显微镜表征胶束薄膜中的排斥力和表面变形
在这里,我们研究了原子力显微镜(AFM)尖端接近胶束表面活性剂膜时的力如何变化,并使用此信息来识别膜的表面结构和杨氏模量。使用10 mM十二烷基硫酸钠(SDS)在石墨界面上产生成排的蠕虫状半胶束。我们发现,氮化硅尖端接近表面时的排斥力呈指数级,衰减长度为2.0±0.1 nm。发现添加Na 2 SO 4会引起这种行为的改变,并且在浓度高于1 mM时清晰地分成两个指数区域。我们还观察到,这些作用力的范围随添加的盐从纯SDS中的〜15 nm增加到Na 2 SO 4下的〜20 nm而增加。浓度为1.34 mM。这些力与静电排斥力不一致,并且被确定为空间位阻。我们表明,在较高盐浓度下的行为与渗透体系中的聚电解质刷的理论是一致的。据此,我们假设表面上存在与吸附的聚合物链相似的胶束刷。另外,使用Sneddon模型从界面附近的数据获取膜的杨氏模量,发现为80±40MPa。在添加氯化镁的情况下,用10 mM十二烷基胺盐酸盐(DAH)溶液进行了类似的实验。发现纯DAH溶液的衰减长度为2.6±0.3 nm,并添加了1.34 mM的MgCl 2使它增加到3.7±0.3 nm。对于DAH,未观察到衰变长度分裂。我们得出的结论是,表面的行为类似于不带电的聚合物刷,因为DAH的离子和表面电荷密度远低于SDS。















































 京公网安备 11010802027423号
京公网安备 11010802027423号