当前位置:
X-MOL 学术
›
ACS Catal.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Structural Dynamics and Evolution of Bismuth Electrodes during Electrochemical Reduction of CO2 in Imidazolium-Based Ionic Liquid Solutions
ACS Catalysis ( IF 11.3 ) Pub Date : 2017-09-26 00:00:00 , DOI: 10.1021/acscatal.7b01370 Jonnathan Medina-Ramos 1 , Sang Soo Lee 1 , Timothy T. Fister 1 , Aude A. Hubaud 1 , Robert L. Sacci , David R. Mullins , John L. DiMeglio 2 , Rachel C. Pupillo 2 , Stephanie M. Velardo 2 , Daniel A. Lutterman , Joel Rosenthal 2 , Paul Fenter 1
ACS Catalysis ( IF 11.3 ) Pub Date : 2017-09-26 00:00:00 , DOI: 10.1021/acscatal.7b01370 Jonnathan Medina-Ramos 1 , Sang Soo Lee 1 , Timothy T. Fister 1 , Aude A. Hubaud 1 , Robert L. Sacci , David R. Mullins , John L. DiMeglio 2 , Rachel C. Pupillo 2 , Stephanie M. Velardo 2 , Daniel A. Lutterman , Joel Rosenthal 2 , Paul Fenter 1
Affiliation
|
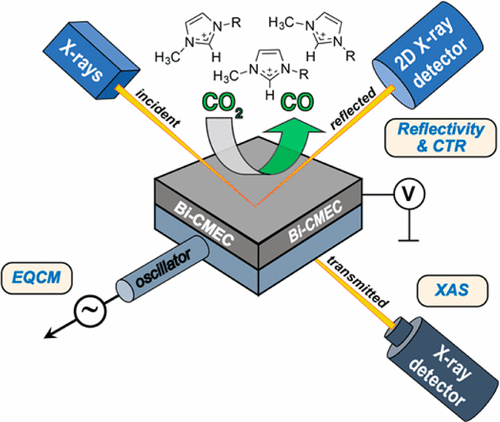
Real-time changes in the composition and structure of bismuth electrodes used for catalytic conversion of CO2 into CO were examined via X-ray absorption spectroscopy (including XANES and EXAFS), electrochemical quartz crystal microbalance (EQCM), and in situ X-ray reflectivity (XR). Measurements were performed with bismuth electrodes immersed in acetonitrile (MeCN) solutions containing a 1-butyl-3-methylimidazolium ([BMIM]+) ionic liquid promoter or electrochemically inactive tetrabutylammonium supporting electrolytes (TBAPF6 and TBAOTf). Altogether, these measurements show that bismuth electrodes are originally a mixture of bismuth oxides (including Bi2O3) and metallic bismuth (Bi0) and that the reduction of oxidized bismuth species to Bi0 is fully achieved under potentials at which CO2 activation takes place. Furthermore, EQCM measurements conducted during cyclic voltammetry revealed that a bismuth-coated quartz crystal exhibits significant shifts in resistance (ΔR) prior to the onset of CO2 reduction near −1.75 V vs Ag/AgCl and pronounced hysteresis in frequency (Δf) and ΔR, which suggests significant changes in roughness or viscosity at the Bi/[BMIM]+ solution interface. In situ XR performed on rhombohedral Bi (001) oriented films indicates that extensive restructuring of the bismuth film cathodes takes place upon polarization to potentials more negative than −1.6 V vs Ag/AgCl, which is characterized by a decrease of the Bi (001) Bragg peak intensity of ≥50% in [BMIM]OTf solutions in the presence and absence of CO2. Over 90% of the reflectivity is recovered during the anodic half-scan, suggesting that the structural changes are mostly reversible. In contrast, such a phenomenon is not observed for thin Bi (001) oriented films in solutions of tetrabutylammonium salts that do not promote CO2 reduction. Overall, these results highlight that Bi electrodes undergo significant potential-dependent chemical and structural transformations in the presence of [BMIM]+-based electrolytes, including the reduction of bismuth oxide to bismuth metal and changes in roughness and near-surface viscosity.
中文翻译:

咪唑鎓离子液体溶液中CO 2电化学还原过程中铋电极的结构动力学和演化
通过X射线吸收光谱法(包括XANES和EXAFS),电化学石英晶体微天平(EQCM)和原位X射线检查了用于将CO 2催化转化为CO的铋电极的组成和结构的实时变化。反射率(XR)。用铋电极浸入含有1-丁基-3-甲基咪唑鎓([BMIM] +)离子液体促进剂或电化学惰性的四丁基铵支持电解质(TBAPF 6和TBAOTf)的乙腈(MeCN)溶液中进行测量。总而言之,这些测量结果表明,铋电极最初是氧化铋(包括Bi 2 O 3)和金属铋(Bi 0),并且在发生CO 2活化的电势下,可以完全实现将氧化铋物种还原为Bi 0。此外,在循环伏安法过程中进行的EQCM测量表明,铋涂层的石英晶体在开始发生CO 2降低之前(相对于Ag / AgCl接近-1.75 V ),电阻(ΔR)发生了显着变化,并且出现了明显的频率滞后(Δf)。和ΔR,表明在Bi / [BMIM] +下,粗糙度或粘度发生了显着变化解决方案界面。在菱形Bi(001)取向薄膜上进行的原位XR分析表明,铋薄膜阴极在极化时发生了广泛的重组,其电位比与Ag / AgCl相比-1.6 V负,这是Bi(001)降低的特征。在存在和不存在CO 2的情况下,在[BMIM] OTf溶液中的布拉格峰强度≥50%。在阳极半扫描期间,超过90%的反射率得以恢复,这表明结构变化大部分是可逆的。相反,对于在不促进CO 2的四丁基铵盐溶液中的Bi(001)取向薄膜来说,却没有观察到这种现象。减少。总体而言,这些结果表明,在[BMIM] +基电解质的存在下,Bi电极会发生明显的电势依赖性化学和结构转变,包括氧化铋还原为铋金属以及粗糙度和近表面粘度的变化。
更新日期:2017-09-26
中文翻译:

咪唑鎓离子液体溶液中CO 2电化学还原过程中铋电极的结构动力学和演化
通过X射线吸收光谱法(包括XANES和EXAFS),电化学石英晶体微天平(EQCM)和原位X射线检查了用于将CO 2催化转化为CO的铋电极的组成和结构的实时变化。反射率(XR)。用铋电极浸入含有1-丁基-3-甲基咪唑鎓([BMIM] +)离子液体促进剂或电化学惰性的四丁基铵支持电解质(TBAPF 6和TBAOTf)的乙腈(MeCN)溶液中进行测量。总而言之,这些测量结果表明,铋电极最初是氧化铋(包括Bi 2 O 3)和金属铋(Bi 0),并且在发生CO 2活化的电势下,可以完全实现将氧化铋物种还原为Bi 0。此外,在循环伏安法过程中进行的EQCM测量表明,铋涂层的石英晶体在开始发生CO 2降低之前(相对于Ag / AgCl接近-1.75 V ),电阻(ΔR)发生了显着变化,并且出现了明显的频率滞后(Δf)。和ΔR,表明在Bi / [BMIM] +下,粗糙度或粘度发生了显着变化解决方案界面。在菱形Bi(001)取向薄膜上进行的原位XR分析表明,铋薄膜阴极在极化时发生了广泛的重组,其电位比与Ag / AgCl相比-1.6 V负,这是Bi(001)降低的特征。在存在和不存在CO 2的情况下,在[BMIM] OTf溶液中的布拉格峰强度≥50%。在阳极半扫描期间,超过90%的反射率得以恢复,这表明结构变化大部分是可逆的。相反,对于在不促进CO 2的四丁基铵盐溶液中的Bi(001)取向薄膜来说,却没有观察到这种现象。减少。总体而言,这些结果表明,在[BMIM] +基电解质的存在下,Bi电极会发生明显的电势依赖性化学和结构转变,包括氧化铋还原为铋金属以及粗糙度和近表面粘度的变化。

































 京公网安备 11010802027423号
京公网安备 11010802027423号