Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Impact of Surface Polyethylene Glycol (PEG) Density on Biodegradable Nanoparticle Transport in Mucus ex Vivo and Distribution in Vivo
ACS Nano ( IF 15.8 ) Pub Date : 2015-08-31 00:00:00 , DOI: 10.1021/acsnano.5b03876 Qingguo Xu 1, 2 , Laura M. Ensign 1, 2, 3 , Nicholas J. Boylan 2, 3 , Arne Schön 4 , Xiaoqun Gong 1, 2, 5, 6 , Jeh-Chang Yang 3 , Nicholas W. Lamb 1, 2 , Shutian Cai 3 , Tao Yu 2, 7 , Ernesto Freire 4 , Justin Hanes 1, 2, 3, 7, 8
ACS Nano ( IF 15.8 ) Pub Date : 2015-08-31 00:00:00 , DOI: 10.1021/acsnano.5b03876 Qingguo Xu 1, 2 , Laura M. Ensign 1, 2, 3 , Nicholas J. Boylan 2, 3 , Arne Schön 4 , Xiaoqun Gong 1, 2, 5, 6 , Jeh-Chang Yang 3 , Nicholas W. Lamb 1, 2 , Shutian Cai 3 , Tao Yu 2, 7 , Ernesto Freire 4 , Justin Hanes 1, 2, 3, 7, 8
Affiliation
|
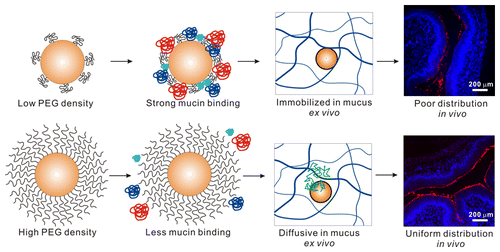
Achieving sustained drug delivery to mucosal surfaces is a major challenge due to the presence of the protective mucus layer that serves to trap and rapidly remove foreign particulates. Nanoparticles engineered to rapidly penetrate mucosal barriers (mucus-penetrating particles, “MPP”) have shown promise for improving drug distribution, retention and efficacy at mucosal surfaces. MPP are densely coated with polyethylene glycol (PEG), which shields the nanoparticle core from adhesive interactions with mucus. However, the PEG density required to impart the “stealth” properties to nanoparticles in mucus, and thus, uniform distribution in vivo, is still unknown. We prepared biodegradable poly(lactic-co-glycolic acid) (PLGA) nanoparticles with a range of PEG surface densities by blending various ratios of a diblock copolymer of PLGA and 5 kDa poly(ethylene glycol) (PLGA–PEG5k) with PLGA. We then evaluated the impact of PEG surface density, measured using an 1H NMR method, on mucin binding in vitro, nanoparticle transport in freshly obtained human cervicovaginal mucus (CVM) ex vivo, and nanoparticle distribution in the mouse cervicovaginal tract in vivo. We found that at least 5% PEG was required to effectively shield the nanoparticle core from interacting with mucus components in vitro and ex vivo, thus leading to enhanced nanoparticle distribution throughout the mouse vagina in vivo. We then demonstrated that biodegradable MPP could be formulated from blends of PLGA and PLGA–PEG polymers of various molecular weights, and that these MPP provide tunable drug loading and drug release rates and durations. Overall, we describe a methodology for rationally designing biodegradable, drug-loaded MPP for more uniform delivery to the vagina.
中文翻译:

生物降解纳米运输粘液表面的聚乙二醇(PEG)密度的影响离体和分布在体内
由于存在用于捕获和快速去除外来微粒的保护性粘液层,因此实现将药物持续递送至粘膜表面是一个重大挑战。经过工程设计可快速穿透粘膜屏障的纳米颗粒(粘液穿透颗粒,“ MPP”)已显示出有望改善在粘膜表面的药物分布,保留和功效的希望。MPP涂有聚乙二醇(PEG)致密涂层,该涂层可保护纳米颗粒核免于与粘液的胶粘剂相互作用。然而,将粘液赋予纳米颗粒“隐身”特性所需的PEG密度,因此在体内的均匀分布仍是未知的。我们准备了可生物降解聚(乳酸-共通过将PLGA和5 kDa聚乙二醇(PLGA–PEG 5k)的二嵌段共聚物的各种比例与PLGA混合,可以得到具有各种PEG表面密度的(-乙醇酸)(PLGA)纳米粒子。然后我们评价了PEG表面密度的影响,测量使用1 1 H NMR方法中,在结合粘蛋白在体外,在新鲜获得的人宫颈粘液的纳米颗粒的运输(CVM)离体,和纳米颗粒分布在小鼠宫颈道体内。我们发现至少需要5%的PEG才能有效地屏蔽纳米颗粒核心,使其在体外和离体时均不与粘液成分相互作用。,因此导致增强纳米粒子在整个小鼠阴道体内的分布。然后,我们证明了可生物降解的MPP可以由各种分子量的PLGA和PLGA-PEG聚合物的共混物配制而成,并且这些MPP提供了可调节的载药量,药物释放速率和持续时间。总体而言,我们描述了一种合理设计可生物降解的,载有药物的MPP以便更均匀地输送至阴道的方法。
更新日期:2015-08-31
中文翻译:

生物降解纳米运输粘液表面的聚乙二醇(PEG)密度的影响离体和分布在体内
由于存在用于捕获和快速去除外来微粒的保护性粘液层,因此实现将药物持续递送至粘膜表面是一个重大挑战。经过工程设计可快速穿透粘膜屏障的纳米颗粒(粘液穿透颗粒,“ MPP”)已显示出有望改善在粘膜表面的药物分布,保留和功效的希望。MPP涂有聚乙二醇(PEG)致密涂层,该涂层可保护纳米颗粒核免于与粘液的胶粘剂相互作用。然而,将粘液赋予纳米颗粒“隐身”特性所需的PEG密度,因此在体内的均匀分布仍是未知的。我们准备了可生物降解聚(乳酸-共通过将PLGA和5 kDa聚乙二醇(PLGA–PEG 5k)的二嵌段共聚物的各种比例与PLGA混合,可以得到具有各种PEG表面密度的(-乙醇酸)(PLGA)纳米粒子。然后我们评价了PEG表面密度的影响,测量使用1 1 H NMR方法中,在结合粘蛋白在体外,在新鲜获得的人宫颈粘液的纳米颗粒的运输(CVM)离体,和纳米颗粒分布在小鼠宫颈道体内。我们发现至少需要5%的PEG才能有效地屏蔽纳米颗粒核心,使其在体外和离体时均不与粘液成分相互作用。,因此导致增强纳米粒子在整个小鼠阴道体内的分布。然后,我们证明了可生物降解的MPP可以由各种分子量的PLGA和PLGA-PEG聚合物的共混物配制而成,并且这些MPP提供了可调节的载药量,药物释放速率和持续时间。总体而言,我们描述了一种合理设计可生物降解的,载有药物的MPP以便更均匀地输送至阴道的方法。































 京公网安备 11010802027423号
京公网安备 11010802027423号