当前位置:
X-MOL 学术
›
Org. Lett.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Approach toward the Understanding of Coupling Mechanism for EDC Reagent in Solvent-Free Mechanosynthesis
Organic Letters ( IF 4.9 ) Pub Date : 2017-09-22 00:00:00 , DOI: 10.1021/acs.orglett.7b02637 Aneta Wróblewska 1 , Piotr Paluch 1 , Ewelina Wielgus 1 , Grzegorz Bujacz 2 , Marta K. Dudek 1 , Marek J. Potrzebowski 1
Organic Letters ( IF 4.9 ) Pub Date : 2017-09-22 00:00:00 , DOI: 10.1021/acs.orglett.7b02637 Aneta Wróblewska 1 , Piotr Paluch 1 , Ewelina Wielgus 1 , Grzegorz Bujacz 2 , Marta K. Dudek 1 , Marek J. Potrzebowski 1
Affiliation
|
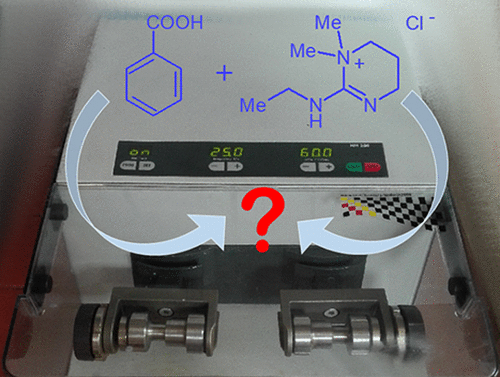
A unique approach in mechanosynthesis, joining solid-state NMR spectroscopy, X-ray crystallography, and theoretical calculations, is employed for the first time to study the mechanism of the formation of the C–N amide bond using EDC·HCl as a coupling reagent. It has been proved that EDC·HCl, which in the crystal lattice exists exclusively in the cyclic form (X-ray data), easily undergoes transformation to a pseudocyclic stable intermediate in reaction with carboxylic acid forming a low-melt phase (differential scanning calorimetry, solid-state NMR). The obtained intermediate is reactive and can be further used for synthesis of amides in reaction with appropriate amines.
中文翻译:

理解无溶剂机械合成中EDC试剂偶联机理的方法
首次采用独特的机械合成方法,结合固态NMR光谱,X射线晶体学和理论计算,以EDC·HCl作为偶联剂研究了C–N酰胺键的形成机理。 。业已证明,在晶格中仅以环状形式存在的EDC·HCl(X射线数据)在与羧酸反应形成低熔点相时,很容易转变为假环稳定中间体(差示扫描量热法) ,固态NMR)。所获得的中间体是反应性的,并且可以在与适当的胺反应中进一步用于酰胺的合成。
更新日期:2017-09-22
中文翻译:

理解无溶剂机械合成中EDC试剂偶联机理的方法
首次采用独特的机械合成方法,结合固态NMR光谱,X射线晶体学和理论计算,以EDC·HCl作为偶联剂研究了C–N酰胺键的形成机理。 。业已证明,在晶格中仅以环状形式存在的EDC·HCl(X射线数据)在与羧酸反应形成低熔点相时,很容易转变为假环稳定中间体(差示扫描量热法) ,固态NMR)。所获得的中间体是反应性的,并且可以在与适当的胺反应中进一步用于酰胺的合成。































 京公网安备 11010802027423号
京公网安备 11010802027423号