当前位置:
X-MOL 学术
›
Bioconjugate Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Attachment Site Cysteine Thiol pKa Is a Key Driver for Site-Dependent Stability of THIOMAB Antibody–Drug Conjugates
Bioconjugate Chemistry ( IF 4.0 ) Pub Date : 2017-09-22 00:00:00 , DOI: 10.1021/acs.bioconjchem.7b00365 Breanna S. Vollmar 1 , Binqing Wei 1 , Rachana Ohri 1 , Jianhui Zhou 1 , Jintang He 1 , Shang-Fan Yu 1 , Douglas Leipold 1 , Ely Cosino 1 , Sharon Yee 1 , Aimee Fourie-O’Donohue 1 , Guangmin Li 1 , Gail L. Phillips 1 , Katherine R. Kozak 1 , Amrita Kamath 1 , Keyang Xu 1 , Genee Lee 1 , Greg A. Lazar 1 , Hans K. Erickson 1
Bioconjugate Chemistry ( IF 4.0 ) Pub Date : 2017-09-22 00:00:00 , DOI: 10.1021/acs.bioconjchem.7b00365 Breanna S. Vollmar 1 , Binqing Wei 1 , Rachana Ohri 1 , Jianhui Zhou 1 , Jintang He 1 , Shang-Fan Yu 1 , Douglas Leipold 1 , Ely Cosino 1 , Sharon Yee 1 , Aimee Fourie-O’Donohue 1 , Guangmin Li 1 , Gail L. Phillips 1 , Katherine R. Kozak 1 , Amrita Kamath 1 , Keyang Xu 1 , Genee Lee 1 , Greg A. Lazar 1 , Hans K. Erickson 1
Affiliation
|
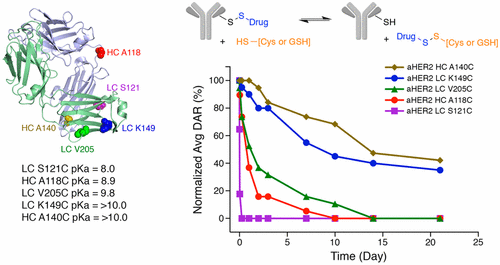
The incorporation of cysteines into antibodies by mutagenesis allows for the direct conjugation of small molecules to specific sites on the antibody via disulfide bonds. The stability of the disulfide bond linkage between the small molecule and the antibody is highly dependent on the location of the engineered cysteine in either the heavy chain (HC) or the light chain (LC) of the antibody. Here, we explore the basis for this site-dependent stability. We evaluated the in vivo efficacy and pharmacokinetics of five different cysteine mutants of trastuzumab conjugated to a pyrrolobenzodiazepine (PBD) via disulfide bonds. A significant correlation was observed between disulfide stability and efficacy for the conjugates. We hypothesized that the observed site-dependent stability of the disulfide-linked conjugates could be due to differences in the attachment site cysteine thiol pKa. We measured the cysteine thiol pKa using isothermal titration calorimetry (ITC) and found that the variants with the highest thiol pKa (LC K149C and HC A140C) were found to yield the conjugates with the greatest in vivo stability. Guided by homology modeling, we identified several mutations adjacent to LC K149C that reduced the cysteine thiol pKa and, thus, decreased the in vivo stability of the disulfide-linked PBD conjugated to LC K149C. We also present results suggesting that the high thiol pKa of LC K149C is responsible for the sustained circulation stability of LC K149C TDCs utilizing a maleimide-based linker. Taken together, our results provide evidence that the site-dependent stability of cys-engineered antibody-drug conjugates may be explained by interactions between the engineered cysteine and the local protein environment that serves to modulate the side-chain thiol pKa. The influence of cysteine thiol pKa on stability and efficacy offers a new parameter for the optimization of ADCs that utilize cysteine engineering.
中文翻译:

附着位点半胱氨酸硫醇p K a是THIOMAB抗体-药物缀合物的位点依赖性稳定性的关键驱动力
通过诱变将半胱氨酸掺入抗体允许小分子通过二硫键直接缀合至抗体的特定位点。小分子与抗体之间的二硫键连接的稳定性高度依赖于工程改造的半胱氨酸在抗体的重链(HC)或轻链(LC)中的位置。在这里,我们探索了这种依赖于站点的稳定性的基础。我们评估了通过二硫键与吡咯并苯并二氮杂(PBD)共轭的曲妥珠单抗的五个不同半胱氨酸突变体的体内功效和药代动力学。观察到二硫键的稳定性和结合物的功效之间存在显着的相关性。ķ一个。我们使用等温滴定量热法(ITC)测量了半胱氨酸硫醇p K a,发现具有最高硫醇p K a的变体(LC K149C和HC A140C)被发现可产生具有最大体内稳定性的结合物。在同源性建模的指导下,我们确定了与LC K149C相邻的几个突变,这些突变降低了半胱氨酸硫醇p K a,从而降低了与LC K149C缀合的二硫键连接的PBD的体内稳定性。我们还提出了表明高硫醇p K a的结果。使用基于马来酰亚胺的接头,LC K149C的TSC负责LC K149C TDC的持续循环稳定性。综上所述,我们的结果提供了证据,即半胱氨酸工程化抗体-药物偶联物的位点依赖性稳定性可以通过工程化半胱氨酸与用于调节侧链硫醇p K a的局部蛋白质环境之间的相互作用来解释。半胱氨酸硫醇p K a对稳定性和功效的影响为利用半胱氨酸工程技术优化ADC提供了新的参数。
更新日期:2017-09-23
中文翻译:

附着位点半胱氨酸硫醇p K a是THIOMAB抗体-药物缀合物的位点依赖性稳定性的关键驱动力
通过诱变将半胱氨酸掺入抗体允许小分子通过二硫键直接缀合至抗体的特定位点。小分子与抗体之间的二硫键连接的稳定性高度依赖于工程改造的半胱氨酸在抗体的重链(HC)或轻链(LC)中的位置。在这里,我们探索了这种依赖于站点的稳定性的基础。我们评估了通过二硫键与吡咯并苯并二氮杂(PBD)共轭的曲妥珠单抗的五个不同半胱氨酸突变体的体内功效和药代动力学。观察到二硫键的稳定性和结合物的功效之间存在显着的相关性。ķ一个。我们使用等温滴定量热法(ITC)测量了半胱氨酸硫醇p K a,发现具有最高硫醇p K a的变体(LC K149C和HC A140C)被发现可产生具有最大体内稳定性的结合物。在同源性建模的指导下,我们确定了与LC K149C相邻的几个突变,这些突变降低了半胱氨酸硫醇p K a,从而降低了与LC K149C缀合的二硫键连接的PBD的体内稳定性。我们还提出了表明高硫醇p K a的结果。使用基于马来酰亚胺的接头,LC K149C的TSC负责LC K149C TDC的持续循环稳定性。综上所述,我们的结果提供了证据,即半胱氨酸工程化抗体-药物偶联物的位点依赖性稳定性可以通过工程化半胱氨酸与用于调节侧链硫醇p K a的局部蛋白质环境之间的相互作用来解释。半胱氨酸硫醇p K a对稳定性和功效的影响为利用半胱氨酸工程技术优化ADC提供了新的参数。






























 京公网安备 11010802027423号
京公网安备 11010802027423号