Journal of Fluorine Chemistry ( IF 1.7 ) Pub Date : 2017-08-18 , DOI: 10.1016/j.jfluchem.2017.08.007 Jie Liu , Jorge R. Barrio , Nagichettiar Satyamurthy
|
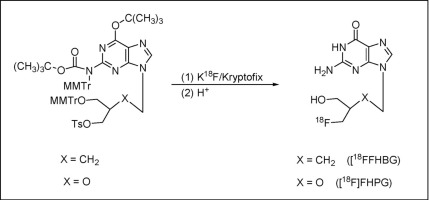
A new, high radiochemical yield synthesis of [18F]FHBG and [18F]FHPG, the most popular imaging agents currently in use for monitoring gene therapy using positron emission tomography (PET), is reported in this work. Protection of sensitive sites in the precursors generally utilized for the preparation of [18F]FHBG and [18F]FHPG using the nucleophilic 18F-fluorination reaction was found to be critical for good radiochemical yields, reliability and reproducibility of the synthetic process. As an initial approach, protection at O6-oxygen in the guanine moiety of the currently used monomethoxytrityl-protected penciclovir tosylate derivative 9 with carbamoyl groups was carried out. Subsequently, full protection of both O6-oxygen and N2-nitrogen in the monomethoxytrityl-protected penciclovir and ganciclovir tosylate analogs 9 and 10 were achieved by their reaction with di-tert-butyl dicarbonate, which resulted in O6-tert-butyl-N2-Boc-monomethoxytrityl-protected penciclovir tosylate 18 and O6-tert-butyl-N2-Boc-monomethoxytrityl-protected ganciclovir tosylate 19, respectively. The newly synthesized carbamoyl- and the Boc- protected precursors were first reacted with non-radioactive KF complexed with Kryptofix 222 to isolate the fluorinated products. Acid hydrolysis of the purified fluorinated intermediates provided the nucleosides FHBG and FHPG. Full characterization of the new precursors as well as the products obtained by fluorination and hydrolysis reactions were carried out by one- and two-dimensional NMR spectroscopy and high resolution mass spectrometry. Single crystal X-ray crystallographic analysis of a model ganciclovir analog 22 confirmed the structural characterization of the new Boc-protected tosylate precursors 18 and 19 by NMR spectroscopy. The carbamoyl- and the Boc-protected precursors were further subjected to radiofluorination followed by acid hydrolysis reactions to furnish [18F]FHBG and [18F]FHPG reliably and reproducibly in excellent radiochemical yields (>65%), much higher than those previously achieved.
中文翻译:

有效合成9-(4- [ 18 F]氟-3-羟基甲基丁基)鸟嘌呤([ 18 F] FHBG)和9-[(3- [ 18 F]氟-1-羟基-2-丙氧基)甲基]鸟嘌呤([ 18 F] FHPG)
这项工作报道了[ 18 F] FHBG和[ 18 F] FHPG的新的高放射化学收率合成,这是目前正用于监测使用正电子发射断层扫描(PET)进行基因治疗的最流行的成像剂。发现通常使用亲核的18 F-氟化反应来保护制备[ 18 F] FHBG和[ 18 F] FHPG的前体中的敏感位点,对于合成方法的良好放射化学收率,可靠性和可重复性至关重要。最初的方法是在当前使用的单甲氧基三苯甲基保护的喷昔洛韦甲苯磺酸酯衍生物9的鸟嘌呤部分的O 6氧处进行保护。与氨基甲酰基一起进行。随后,两者的充分保护ö 6 -氧和Ñ 2 -氮在单甲氧保护喷昔洛韦和更昔洛韦甲苯磺酸酯类似物9和10,通过它们的反应与二-实现叔丁基二碳酸酯,这导致ö 6 -叔-丁基- ñ 2 -Boc单甲氧保护的喷昔洛韦甲苯磺酸酯18和ö 6 -叔丁基- ñ 2 -Boc单甲氧保护的更昔洛韦甲苯磺酸酯19, 分别。首先将新合成的氨基甲酰基和Boc保护的前体与与Kryptofix 222络合的非放射性KF反应,以分离氟化产物。纯化的氟化中间体的酸水解提供了核苷FHBG和FHPG。通过一维和二维NMR光谱和高分辨率质谱对新的前体以及通过氟化和水解反应获得的产物进行了全面表征。更昔洛韦模型22的单晶X射线晶体学分析证实了新的受Boc保护的甲苯磺酸酯前体18和19的结构特征通过NMR光谱。氨基甲酰基和Boc保护的前体进一步进行放射氟化,然后进行酸水解反应,可可靠且可重复地以优异的放射化学收率(> 65%)提供[ 18 F] FHBG和[ 18 F] FHPG,远高于以前的收率。实现。












































 京公网安备 11010802027423号
京公网安备 11010802027423号