Bioorganic Chemistry ( IF 4.5 ) Pub Date : 2017-07-25 , DOI: 10.1016/j.bioorg.2017.07.012
Madiha Kazmi , Sumera Zaib , Sayyeda Tayyeba Amjad , Imtiaz Khan , Aliya Ibrar , Aamer Saeed , Jamshed Iqbal
|
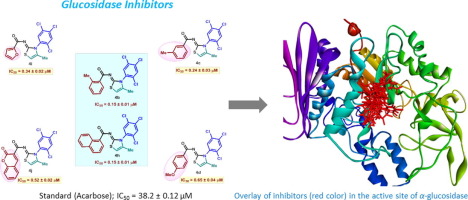
A series of iminothiazolines (4a–j) featuring 2,4,5-trichlorophenyl moiety and aroyl/heteroaroyl substituents has been prepared from readily accessible thioureas. In-vitro screening against glucosidase enzymes showed highly specific inhibition of α-glucosidase with a marked dependence of the potency upon the nature of the aroyl/heteroaroyl substituents. The most potent representatives, bearing ortho-tolyl and bulky naphthyl groups displayed the highest inhibitory potential with IC50 value of 0.15 ± 0.01 µM compared to standard drug acarbose (IC50 = 38.2 ± 0.12 µM). Several other derivatives (4c, 4d, 4i and 4j) were also significantly powerful and selective inhibitors of α-glucosidase. Binding interactions of potent compounds 4b, 4c, 4h and 4i with α-glucosidase were explored by molecular docking simulation. These results clearly identified a new class of structural leads which can be further investigated for the development of promising α-glucosidase inhibitors for the prevention of diabetes mellitus.
中文翻译:

探索以2,4,5-三氯苯基部分为新型有效,选择性和体外有效葡糖苷酶抑制剂的芳酰基/杂芳基亚氨基噻唑啉
一系列iminothiazolines的(图4a-j中设有2,4,5)-三氯苯基部分和芳酰基/杂芳取代基已经从容易获得的硫脲制备。在-体外筛选针对葡萄糖苷酶显示出高度特异性抑制α葡糖苷酶与效力时的芳酰基/杂芳取代基的性质具有显着的依赖性。与标准药物阿卡波糖(IC 50 = 38.2±0.12 µM)相比,带有邻甲苯基和庞大萘基的最有力代表具有最高的抑制潜力,IC 50值为0.15±0.01 µM 。其他几种导数(4c,4d,4i和4j)也是α-葡糖苷酶的显着有效和选择性抑制剂。通过分子对接模拟研究了有效化合物4b,4c,4h和4i与α-葡萄糖苷酶的结合相互作用。这些结果清楚地确定了新的一类结构化的先导,其可进一步用于开发有望用于预防糖尿病的α-葡萄糖苷酶抑制剂。

































 京公网安备 11010802027423号
京公网安备 11010802027423号