当前位置:
X-MOL 学术
›
J. Am. Chem. Soc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Synthesis of Fijiolide A via an Atropselective Paracyclophane Formation
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2015-08-27 , DOI: 10.1021/jacs.5b07964 Christoph Heinz 1 , Nicolai Cramer 1
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2015-08-27 , DOI: 10.1021/jacs.5b07964 Christoph Heinz 1 , Nicolai Cramer 1
Affiliation
|
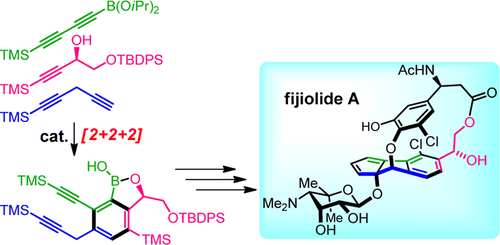
Fijiolide A is a secondary metabolite isolated from a marine-derived actinomycete and displays inhibitory activity against TNF-α-induced activation of NFκB, an important transcription factor and a potential target for the treatment of different cancers and inflammation related diseases. Fijiolide A is a glycosylated complex paracyclophane, which is structurally closely related to the Bergman-aromatization product of enediyne C-1027. We report an enantioselective synthesis of fijiolide A demonstrating the power of fully intermolecular ruthenium-catalyzed [2 + 2 + 2]-cyclotrimerizations with three different alkynes to assemble the heavily substituted central arene core. The characteristic strained [2.6]paracyclophane structure is accessed by a templated atropselective macroetherification reaction.
中文翻译:

通过 Atropselective Paracyclophane 形成合成斐济内酯 A
Fijiolide A 是一种从海洋来源的放线菌中分离出来的次级代谢物,对 TNF-α 诱导的 NFκB 激活具有抑制活性,NFκB 是一种重要的转录因子,是治疗不同癌症和炎症相关疾病的潜在靶点。Fijiolide A 是一种糖基化复合物对环芳烷,在结构上与烯二炔 C-1027 的伯格曼芳香化产物密切相关。我们报告了 fijiolide A 的对映选择性合成,展示了完全分子间钌催化的 [2 + 2 + 2]-环三聚与三种不同的炔烃以组装重取代的中心芳烃核心的能力。特征应变 [2.6] 对环芳烷结构可通过模板化的 atropselective 宏醚化反应获得。
更新日期:2015-08-27
中文翻译:

通过 Atropselective Paracyclophane 形成合成斐济内酯 A
Fijiolide A 是一种从海洋来源的放线菌中分离出来的次级代谢物,对 TNF-α 诱导的 NFκB 激活具有抑制活性,NFκB 是一种重要的转录因子,是治疗不同癌症和炎症相关疾病的潜在靶点。Fijiolide A 是一种糖基化复合物对环芳烷,在结构上与烯二炔 C-1027 的伯格曼芳香化产物密切相关。我们报告了 fijiolide A 的对映选择性合成,展示了完全分子间钌催化的 [2 + 2 + 2]-环三聚与三种不同的炔烃以组装重取代的中心芳烃核心的能力。特征应变 [2.6] 对环芳烷结构可通过模板化的 atropselective 宏醚化反应获得。















































 京公网安备 11010802027423号
京公网安备 11010802027423号