Bioorganic & Medicinal Chemistry ( IF 3.3 ) Pub Date : 2017-09-19 , DOI: 10.1016/j.bmc.2017.09.024 Nataraj S. Pagadala , Trent C. Bjorndahl , Michael Joyce , David S. Wishart , Khajamohiddin Syed , Abdolamir Landi
|
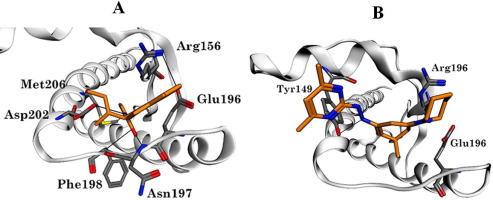
Prion diseases are fatal neurodegenerative disorders of the central nervous system characterized by the accumulation of a protease resistant form (PrPSc) of the cellular prion protein (PrPC) in the brain. Two types of cellular prion (PrPC) compounds have been identified that appear to affect prion conversion are known as Effective Binders (EBs) and Accelerators (ACCs). Effective binders shift the balance in favour of PrPC, whereas Accelerators favour the formation of PrPSc. Molecular docking indicates EBs and ACCs both bind to pocket-D of the SHaPrPC molecule. However, EBs and ACCs may have opposing effects on the stability of the salt bridge between Arg156 and Glu196/Glu200. Computational docking data indicate that the hydrophobic benzamide group of the EB, GFP23 and the 1-(3,3-dimethylcyclohexylidene)piperidinium group of the ACC, GFP22 play an important role in inhibition and conversion from SHaPrPC to SHaPrPSc, respectively. Experimentally, NMR confirmed the amide chemical shift perturbations observed upon the binding of GFP23 to pocket-D of SHaPrPC. Consistent with its role as an ACC, titration of GFP22 resulted in widespread chemical shift changes and signal intensity loss due to protein unfolding. Virtual screening of a ligand database using the molecular scaffold developed from the set of EBs identified six of our compounds (previously studied using fluorescence quenching) as being among the top 100 best binders. Among them, compounds 5 and 6 were found to be particularly potent in decreasing the accumulation SHaPrPSc in ScN2a cells with an IC50 of ∼35 µM and 20 µM.
中文翻译:

化合物(3- {5-[(2,5-二甲氧基苯基)氨基] -1,3,4-噻二唑啉-2-基} -5,8-甲氧基-2 H-铬-2--2- )抑制ion病毒。ScN2a细胞中具有较低IC 50的从PrP C到PrP Sc的蛋白质转化
on病毒疾病是中枢神经系统的致命性神经退行性疾病,其特征是脑中细胞病毒蛋白(PrP C)的蛋白酶抗性形式(PrP Sc)积累。两种类型的细胞朊病毒的(PRP Ç)化合物已经鉴定了表现出影响朊病毒转换被称为有效粘合剂(EBS)和加速器(ACC的)。有效的粘合剂会转移平衡,有利于PrP C,而促进剂则有利于PrP Sc的形成。分子对接表明EB和ACC均与SHaPrP C分子的D口袋结合。但是,EB和ACC可能会对Arg 156之间的盐桥的稳定性产生相反的影响和Glu 196 / Glu 200。计算的对接数据表明,EB,GFP23的疏水苯甲酰胺基团和ACC,GFP22的1-(3,3-二甲基环己叉基)哌啶鎓基团分别在抑制和从SHaPrP C转化为SHaPrP Sc中发挥重要作用。在实验上,NMR证实了GFP23与SHaPrP C的Pocket-D结合后观察到的酰胺化学位移扰动。GFP22的滴定与其作为ACC的作用一致,导致广泛的化学位移变化和由于蛋白质解折叠而引起的信号强度损失。使用从一系列EB分子开发的分子支架对配体数据库进行的虚拟筛选确定了我们的六种化合物(之前使用荧光猝灭进行了研究)是前100种最佳结合剂之一。其中,发现化合物5和6对降低ScN2a细胞中SHaPrP Sc的积累特别有效,IC 50约为35 µM和20 µM。































 京公网安备 11010802027423号
京公网安备 11010802027423号