Chemical Engineering Journal ( IF 13.3 ) Pub Date : 2017-09-20 , DOI: 10.1016/j.cej.2017.09.109
Shangtao Liang , Hui Lin , Xiufen Yan , Qingguo Huang
|
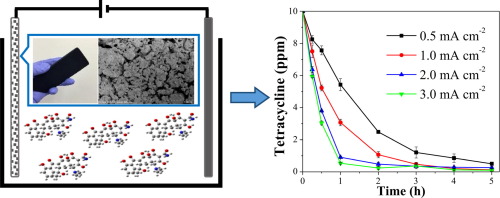
The goal of this study is to evaluate Magnéli phase titanium oxide (Ti4O7) as an anode material for potential application in electrochemical oxidation of organic pollutants in water. The removal of tetracycline (TC) was systematically investigated in terms of kinetics, reaction mechanisms and pathways, and multi-species toxicity. Application of 0.5–3 mA cm−2 current densities resulted in >90% total removal of TC over a wide range of initial concentrations from 1 ppm to 50 ppm with half-lives between 28 mins and 75 mins. The oxidation mechanisms were further elucidated using salicylic acid (SA) as a hydroxyl free radical trap. At least 40% of total TC removal was attributable to reactions mediated by hydroxyl radicals, which were generated on Magnéli phase Ti4O7 at a rate of 2 × 10−9 mol cm−2 min−1 under 0.5 mA cm−2 applied current density. Tests on Escherichia coli culture indicated that electro-oxidation of TC by Magnéli phase Ti4O7 anode successfully reduced the original antimicrobial activity to a level below detection limit. However, for freshwater micro algae Scenedesmus obliquus, inhibitory effects persisted in the first couple of hours and then dramatically reduced during the last stage of treatment, likely due to intermediate products that later mineralized and detoxified. Reaction pathways were proposed based on the data of high-resolution mass spectrometry, and oxidation products with antibiotic potency similar to or greater than TC were identified in 1 h treatment sample, but not detectable in the end-of-treatment solution.
中文翻译:

Magnéli相Ti 4 O 7多孔阳极对四环素的电氧化:动力学,产物和毒性
这项研究的目的是评估Magnéli相氧化钛(Ti 4 O 7)作为阳极材料在水中有机污染物的电化学氧化中的潜在应用。从动力学,反应机理和途径以及多物种毒性方面系统地研究了四环素(TC)的去除。施加0.5–3 mA cm -2的电流密度可使TC在从1 ppm到50 ppm的较宽初始浓度范围内的总去除率达到90%以上,半衰期在28分钟至75分钟之间。使用水杨酸(SA)作为羟基自由基捕获剂进一步阐明了氧化机理。至少40%的总TC去除归因于在Magnéli相Ti上产生的羟基自由基介导的反应在0.5 mA cm -2施加的电流密度下,以2×10 -9 mol cm -2 min -1的速率形成4 O 7。对大肠杆菌培养物的测试表明,Magnéli相Ti 4 O 7阳极对TC的电氧化成功地将原始抗菌活性降低到检测极限以下。然而,对于淡水微藻斜角藻,抑制作用在最初的几个小时内持续存在,然后在治疗的最后阶段急剧降低,这可能是由于中间产物后来被矿化和解毒所致。根据高分辨率质谱数据提出了反应途径,并在1 h处理样品中鉴定出了具有与TC相似或更高TC的抗生素效价的氧化产物,但在处理结束溶液中未检测到。







































 京公网安备 11010802027423号
京公网安备 11010802027423号