Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Organ-specific regulation of ATP7A abundance is coordinated with systemic copper homeostasis.
Scientific Reports ( IF 3.8 ) Pub Date : 2017-Sep-20 , DOI: 10.1038/s41598-017-11961-z
Haarin Chun , Tracy Catterton , Heejeong Kim , Jaekwon Lee , Byung-Eun Kim
Scientific Reports ( IF 3.8 ) Pub Date : 2017-Sep-20 , DOI: 10.1038/s41598-017-11961-z
Haarin Chun , Tracy Catterton , Heejeong Kim , Jaekwon Lee , Byung-Eun Kim
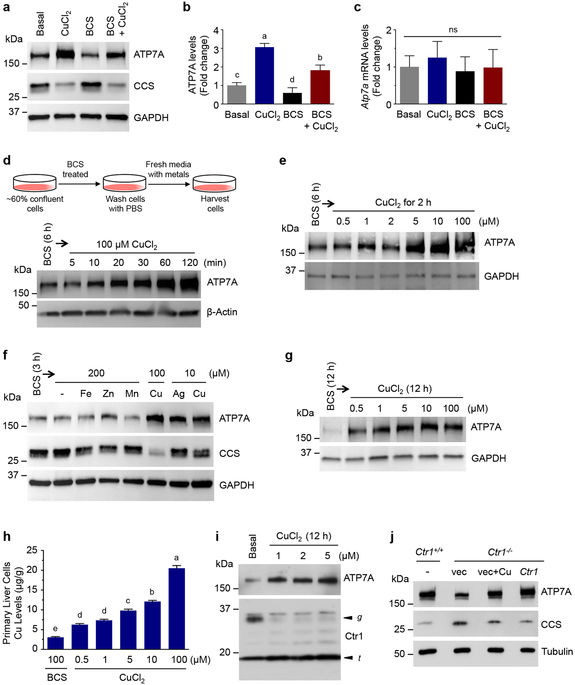
|
Copper (Cu) is an essential cofactor for various enzymatic activities including mitochondrial electron transport, iron mobilization, and peptide hormone maturation. Consequently, Cu dysregulation is associated with fatal neonatal disease, liver and cardiac dysfunction, and anemia. While the Cu transporter ATP7A plays a major role in both intestinal Cu mobilization to the periphery and prevention of Cu over-accumulation, it is unclear how regulation of ATP7A contributes to Cu homeostasis in response to systemic Cu fluctuation. Here we show, using Cu-deficient mouse models, that steady-state levels of ATP7A are lower in peripheral tissues (including the heart, spleen, and liver) under Cu deficiency and that subcutaneous administration of Cu to these animals restore normal ATP7A levels in these tissues. Strikingly, ATP7A in the intestine is regulated in the opposite manner - low systemic Cu increases ATP7A while subcutaneous Cu administration decreases ATP7A suggesting that intestine-specific non-autonomous regulation of ATP7A abundance may serve as a key homeostatic control for Cu export into the circulation. Our results support a systemic model for how a single transporter can be inversely regulated in a tissue-specific manner to maintain organismal Cu homeostasis.
中文翻译:

ATP7A丰度的器官特异性调节与全身铜稳态协调。
铜(Cu)是各种酶活性(包括线粒体电子传输,铁动员和肽激素成熟)必不可少的辅助因子。因此,铜失调与致命的新生儿疾病,肝和心脏功能障碍以及贫血有关。虽然铜转运蛋白ATP7A在肠道铜向周围的动员和防止铜的过度积累中都起着主要作用,但尚不清楚ATP7A的调节如何响应系统性铜的波动而促进铜的稳态。在这里,我们显示出使用铜缺乏的小鼠模型,在缺乏铜的情况下,外周组织(包括心脏,脾脏和肝脏)中的ATP7A稳态水平较低,并且对这些动物进行皮下注射铜可以恢复正常的ATP7A水平。这些组织。惊人地 肠道中的ATP7A以相反的方式进行调节-低全身性铜会增加ATP7A,而皮下施用铜会降低ATP7A,这表明肠道特定的非自治性ATP7A丰度调节可能是铜出口到循环系统的关键稳态方法。我们的结果支持系统模型,该模型可以如何以组织特异性方式逆向调节单个转运蛋白以维持机体铜稳态。
更新日期:2017-09-20
中文翻译:

ATP7A丰度的器官特异性调节与全身铜稳态协调。
铜(Cu)是各种酶活性(包括线粒体电子传输,铁动员和肽激素成熟)必不可少的辅助因子。因此,铜失调与致命的新生儿疾病,肝和心脏功能障碍以及贫血有关。虽然铜转运蛋白ATP7A在肠道铜向周围的动员和防止铜的过度积累中都起着主要作用,但尚不清楚ATP7A的调节如何响应系统性铜的波动而促进铜的稳态。在这里,我们显示出使用铜缺乏的小鼠模型,在缺乏铜的情况下,外周组织(包括心脏,脾脏和肝脏)中的ATP7A稳态水平较低,并且对这些动物进行皮下注射铜可以恢复正常的ATP7A水平。这些组织。惊人地 肠道中的ATP7A以相反的方式进行调节-低全身性铜会增加ATP7A,而皮下施用铜会降低ATP7A,这表明肠道特定的非自治性ATP7A丰度调节可能是铜出口到循环系统的关键稳态方法。我们的结果支持系统模型,该模型可以如何以组织特异性方式逆向调节单个转运蛋白以维持机体铜稳态。

































 京公网安备 11010802027423号
京公网安备 11010802027423号