Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Design, synthesis and structure-activity relationship of 3,6-diaryl-7H-[1,2,4]triazolo[3,4-b][1,3,4]thiadiazines as novel tubulin inhibitors.
Scientific Reports ( IF 3.8 ) Pub Date : 2017-Sep-20 , DOI: 10.1038/s41598-017-10860-7 Qile Xu , Kai Bao , Maolin Sun , Jingwen Xu , Yueting Wang , Haiqiu Tian , Daiying Zuo , Qi Guan , Yingliang Wu , Weige Zhang
Scientific Reports ( IF 3.8 ) Pub Date : 2017-Sep-20 , DOI: 10.1038/s41598-017-10860-7 Qile Xu , Kai Bao , Maolin Sun , Jingwen Xu , Yueting Wang , Haiqiu Tian , Daiying Zuo , Qi Guan , Yingliang Wu , Weige Zhang
|
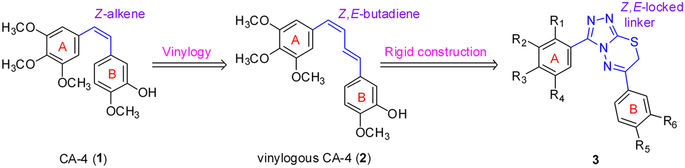
A novel series of 3,6-diaryl-7H-[1,2,4]triazolo[3,4-b][1,3,4]thiadiazines were designed, synthesized and biologically evaluated as vinylogous CA-4 analogues, which involved a rigid [1,2,4]triazolo[3,4-b][1,3,4]thiadiazine scaffold to fix the configuration of (Z,E)-butadiene linker of A-ring and B-ring. Among these rigidly vinylogous CA-4 analogues, compounds 4d, 5b, 5i, 6c, 6e, 6g, 6i and 6k showed excellent antiproliferative activities against SGC-7901, A549 and HT-1080 cell lines with IC50 values at the nanomolar level. Compound 6i showed the most highly active antiproliferative activity against the three human cancer cell lines with an IC50 values of 0.011-0.015 µM, which are comparable to those of CA-4 (IC50 = 0.009-0.013 µM). Interestingly, SAR studies revealed that 3,4-methylenedioxyphenyl, 3,4-dimethoxyphenyl, 3-methoxyphenyl and 4-methoxyphenyl could replace the classic 3,4,5-trimethoxyphenyl in CA-4 structure and keep antiproliferative activity in this series of designed compounds. Tubulin polymerization experiments showed that 6i could effectively inhibit tubulin polymerization, which was corresponded with CA-4, and immunostaining experiments suggested that 6i significantly disrupted microtubule/tubulin dynamics. Furthermore, 6i potently induced cell cycle arrest at G2/M phase in SGC-7901 cells. Competitive binding assays and docking studies suggested that compound 6i binds to the tubulin perfectly at the colchicine binding site. Taken together, these results revealed that 6i may become a promising lead compound for new anticancer drugs discovery.
中文翻译:

3,6-二芳基-7H- [1,2,4]三唑[3,4-b] [1,3,4]噻二嗪作为新型微管蛋白抑制剂的设计,合成及构效关系。
设计,合成了一系列新颖的3,6-二芳基-7H- [1,2,4]三唑并[3,4-b] [1,3,4]噻二嗪作为乙烯基CA-4类似物,并对其进行了生物学评估。涉及刚性的[1,2,4]三唑并[3,4-b] [1,3,4]噻二嗪支架固定A环和B环的(Z,E)-丁二烯接头的构型。在这些硬质乙烯基CA-4类似物中,化合物4d,5b,5i,6c,6e,6g,6i和6k对SGC-7901,A549和HT-1080细胞系表现出优异的抗增殖活性,其IC 50值为纳摩尔水平。化合物6i对三种人类癌细胞系表现出最高的抗增殖活性,IC 50值为0.011-0.015 µM,与CA-4相当(IC 50 = 0.009-0.013 µM)。有趣的是,SAR研究表明3,4-亚甲基二氧基苯基,3,4-二甲氧基苯基,3-甲氧基苯基和4-甲氧基苯基可以取代CA-4结构中的经典3,4,5-三甲氧基苯基,并在此系列设计中保持抗增殖活性。化合物。微管蛋白聚合实验表明6i可以有效抑制微管蛋白聚合,这与CA-4相对应,免疫染色实验表明6i显着破坏了微管/微管蛋白的动力学。此外,6i有效诱导细胞周期停滞在G 2/ M相在SGC-7901细胞中。竞争性结合测定和对接研究表明,化合物6i在秋水仙碱结合位点上与微管蛋白完美结合。综上所述,这些结果表明6i可能成为新的抗癌药物发现的有前途的先导化合物。
更新日期:2017-09-20
中文翻译:

3,6-二芳基-7H- [1,2,4]三唑[3,4-b] [1,3,4]噻二嗪作为新型微管蛋白抑制剂的设计,合成及构效关系。
设计,合成了一系列新颖的3,6-二芳基-7H- [1,2,4]三唑并[3,4-b] [1,3,4]噻二嗪作为乙烯基CA-4类似物,并对其进行了生物学评估。涉及刚性的[1,2,4]三唑并[3,4-b] [1,3,4]噻二嗪支架固定A环和B环的(Z,E)-丁二烯接头的构型。在这些硬质乙烯基CA-4类似物中,化合物4d,5b,5i,6c,6e,6g,6i和6k对SGC-7901,A549和HT-1080细胞系表现出优异的抗增殖活性,其IC 50值为纳摩尔水平。化合物6i对三种人类癌细胞系表现出最高的抗增殖活性,IC 50值为0.011-0.015 µM,与CA-4相当(IC 50 = 0.009-0.013 µM)。有趣的是,SAR研究表明3,4-亚甲基二氧基苯基,3,4-二甲氧基苯基,3-甲氧基苯基和4-甲氧基苯基可以取代CA-4结构中的经典3,4,5-三甲氧基苯基,并在此系列设计中保持抗增殖活性。化合物。微管蛋白聚合实验表明6i可以有效抑制微管蛋白聚合,这与CA-4相对应,免疫染色实验表明6i显着破坏了微管/微管蛋白的动力学。此外,6i有效诱导细胞周期停滞在G 2/ M相在SGC-7901细胞中。竞争性结合测定和对接研究表明,化合物6i在秋水仙碱结合位点上与微管蛋白完美结合。综上所述,这些结果表明6i可能成为新的抗癌药物发现的有前途的先导化合物。































 京公网安备 11010802027423号
京公网安备 11010802027423号