当前位置:
X-MOL 学术
›
Chem. Mater.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Layered Lepidocrocite Type Structure Isolated by Revisiting the Sol–Gel Chemistry of Anatase TiO2: A New Anode Material for Batteries
Chemistry of Materials ( IF 7.2 ) Pub Date : 2017-09-19 00:00:00 , DOI: 10.1021/acs.chemmater.7b02674
Jiwei Ma 1 , Kyle G. Reeves 1 , Ana-Gabriela Porras Gutierrez 1 , Monique Body 2 , Christophe Legein 2 , Katsuyoshi Kakinuma 3 , Olaf J. Borkiewicz 4 , Karena W. Chapman 4 , Henri Groult 1 , Mathieu Salanne 1, 5 , Damien Dambournet 1, 5
Chemistry of Materials ( IF 7.2 ) Pub Date : 2017-09-19 00:00:00 , DOI: 10.1021/acs.chemmater.7b02674
Jiwei Ma 1 , Kyle G. Reeves 1 , Ana-Gabriela Porras Gutierrez 1 , Monique Body 2 , Christophe Legein 2 , Katsuyoshi Kakinuma 3 , Olaf J. Borkiewicz 4 , Karena W. Chapman 4 , Henri Groult 1 , Mathieu Salanne 1, 5 , Damien Dambournet 1, 5
Affiliation
|
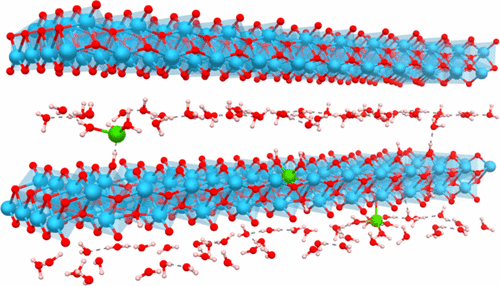
Searches for new electrode materials for batteries must take into account financial and environmental costs to be useful in practical devices. The sol–gel chemistry has been widely used to design and implement new concepts for the emergence of advanced materials such as hydride organic–inorganic composites. Here, we show that the simple reaction system including titanium alkoxide and water can be used to stabilize a new class of electrode materials. By investigating the crystallization path of anatase TiO2, an X-ray amorphous intermediate phase has been identified whose local structure probed by the pair distribution function, 1H solid-state NMR and density functional theory (DFT) calculations, consists of a layered type structure as found in the lepidocrocite. This phase presents the following general formula Ti2–x□xO4–4x(OH)4x·nH2O (x ∼ 0.5) where the substitution of oxide by hydroxide anions leads to the formation of titanium vacancies (□) and H2O molecules are located in interlayers. Solid-state 1H NMR has enabled us to characterize three main hydroxide environments, Ti□–OH, Ti2□2–OH, and Ti3□–OH, and layered H2O molecules. The electrochemical properties of this phase were investigated vs lithium and were shown to be very promising with reversible capacities of around 200 mAh·g–1 and an operating voltage of 1.55 V. We further showed that the lithium intercalation proceeds via a solid-solution mechanism. 7Li solid-state NMR and DFT calculations allowed us to identify lithium host sites that are located at the titanium vacancies and interlayer space with lithium being solvated by structural water molecules. The easy fabrication, the absence of lithium, easier recycling, and the encouraging properties make this class of materials very attractive for competitive electrodes for batteries. We thus demonstrate that revisiting an “old” chemistry with advanced characterization tools allows one to discover new materials of technological relevance.
中文翻译:

通过重新研究锐钛矿型TiO 2的溶胶-凝胶化学来分离层状细铁矿型结构:一种新型的电池负极材料
寻找新的电池电极材料必须考虑财务和环境成本,以在实际设备中有用。溶胶-凝胶化学已被广泛用于设计和实施新概念,以实现诸如氢化物有机-无机复合材料之类的高级材料的出现。在这里,我们表明,包括醇钛和水在内的简单反应体系可用于稳定新型电极材料。通过研究锐钛矿型TiO 2的结晶路径,已鉴定出一种X射线无定形中间相,该中间相的局部结构由对分布函数1探测。H固态NMR和密度泛函理论(DFT)的计算由在纤铁矿中发现的层状结构组成。这一阶段具有以下通式Ti 2- X □ X ø 4-4 X(OH)4 X · Ñ ħ 2 O(X〜0.5),其中氧化物的由氢氧根阴离子导致钛空位的形成取代(□ )和H 2 O分子位于中间层。固态1 H NMR使我们能够表征三种主要的氢氧化物环境,Ti□-OH,Ti 2 □ 2 -OH和Ti 3 □-OH,以及层状H2 O分子。研究了该相相对于锂的电化学性质,并显示出非常有前途的可逆容量,其可逆容量约为200 mAh·g –1,工作电压为1.55V。我们进一步表明,锂的插入是通过固溶机理进行的。7Li固态NMR和DFT计算使我们能够确定位于钛空位和层间空间的锂主体位点,其中锂被结构水分子溶剂化。易于制造,不含锂,更易于回收利用和令人鼓舞的特性,使得此类材料对于电池用竞争性电极极具吸引力。因此,我们证明,使用先进的表征工具重新审视“旧”化学物质可以发现具有技术意义的新材料。
更新日期:2017-09-20
中文翻译:

通过重新研究锐钛矿型TiO 2的溶胶-凝胶化学来分离层状细铁矿型结构:一种新型的电池负极材料
寻找新的电池电极材料必须考虑财务和环境成本,以在实际设备中有用。溶胶-凝胶化学已被广泛用于设计和实施新概念,以实现诸如氢化物有机-无机复合材料之类的高级材料的出现。在这里,我们表明,包括醇钛和水在内的简单反应体系可用于稳定新型电极材料。通过研究锐钛矿型TiO 2的结晶路径,已鉴定出一种X射线无定形中间相,该中间相的局部结构由对分布函数1探测。H固态NMR和密度泛函理论(DFT)的计算由在纤铁矿中发现的层状结构组成。这一阶段具有以下通式Ti 2- X □ X ø 4-4 X(OH)4 X · Ñ ħ 2 O(X〜0.5),其中氧化物的由氢氧根阴离子导致钛空位的形成取代(□ )和H 2 O分子位于中间层。固态1 H NMR使我们能够表征三种主要的氢氧化物环境,Ti□-OH,Ti 2 □ 2 -OH和Ti 3 □-OH,以及层状H2 O分子。研究了该相相对于锂的电化学性质,并显示出非常有前途的可逆容量,其可逆容量约为200 mAh·g –1,工作电压为1.55V。我们进一步表明,锂的插入是通过固溶机理进行的。7Li固态NMR和DFT计算使我们能够确定位于钛空位和层间空间的锂主体位点,其中锂被结构水分子溶剂化。易于制造,不含锂,更易于回收利用和令人鼓舞的特性,使得此类材料对于电池用竞争性电极极具吸引力。因此,我们证明,使用先进的表征工具重新审视“旧”化学物质可以发现具有技术意义的新材料。



































 京公网安备 11010802027423号
京公网安备 11010802027423号