当前位置:
X-MOL 学术
›
Biomacromolecules
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
A Polymer Therapeutic Having Universal Heparin Reversal Activity: Molecular Design and Functional Mechanism
Biomacromolecules ( IF 5.5 ) Pub Date : 2017-09-19 00:00:00 , DOI: 10.1021/acs.biomac.7b00994
Manu Thomas Kalathottukaren 1 , Srinivas Abbina 1 , Kai Yu 1 , Rajesh A. Shenoi 1, 2 , A. Louise Creagh 3 , Charles Haynes 1, 3 , Jayachandran N. Kizhakkedathu 1, 4
Biomacromolecules ( IF 5.5 ) Pub Date : 2017-09-19 00:00:00 , DOI: 10.1021/acs.biomac.7b00994
Manu Thomas Kalathottukaren 1 , Srinivas Abbina 1 , Kai Yu 1 , Rajesh A. Shenoi 1, 2 , A. Louise Creagh 3 , Charles Haynes 1, 3 , Jayachandran N. Kizhakkedathu 1, 4
Affiliation
|
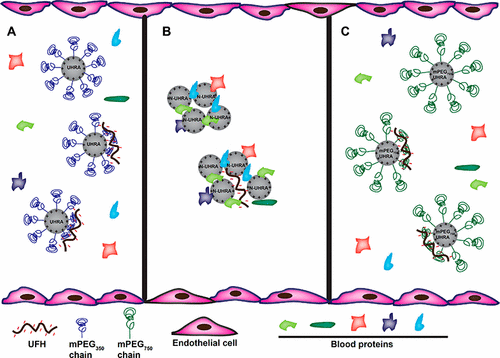
Heparins are widely used to prevent blood clotting during surgeries and for the treatment of thrombosis. However, bleeding associated with heparin therapy is a concern. Protamine, the only approved antidote for unfractionated heparin (UFH) could cause adverse cardiovascular events. Here, we describe a unique molecular design used in the development of a synthetic dendritic polycation named as universal heparin reversal agent (UHRA), an antidote for all clinically used heparin anticoagulants. We elucidate the mechanistic basis for the selectivity of UHRA to heparins and its nontoxic nature. Isothermal titration calorimetry based binding studies of UHRAs having different methoxypolyethylene glycol (mPEG) brush structures with UFH as a function of solution conditions, including ionic strength, revealed that mPEG chains impose entropic penalty to the electrostatic binding. Binding studies confirm that, unlike protamine or N-UHRA (a truncated analogue of UHRA with no mPEG chains), the mPEG chains in UHRA avert nonspecific interactions with blood proteins and provide selectivity toward heparins through a combined steric repulsion and Donnan shielding effect (a balance of Fel and Fsteric). Clotting assays reveal that UHRA with mPEG chains did not adversely affect clotting, and neutralized UFH over a wide range of concentrations. Conversely, N-UHRA and protamine display intrinsic anticoagulant activity and showed a narrow concentration window for UFH neutralization. In addition, we found that mPEG chains regulate the size of antidote-UFH complexes, as revealed by atomic force microscopy and dynamic light scattering studies. UHRA molecules with mPEG chains formed smaller complexes with UFH, compared to N-UHRA and protamine. Finally, fluorescence and ELISA experiments show that UHRA disrupts antithrombin-UFH complexes to neutralize heparin’s activity.
中文翻译:

具有通用肝素逆转活性的聚合物疗法:分子设计和功能机制。
肝素被广泛用于预防手术期间的血液凝结和血栓形成的治疗。然而,与肝素治疗相关的出血是一个问题。鱼精蛋白是唯一批准的普通肝素(UFH)的解毒剂,可能引起不良心血管事件。在这里,我们描述了一种独特的分子设计,用于开发称为通用肝素逆转剂(UHRA)的合成树突状聚阳离子,这是所有临床使用的肝素抗凝剂的解毒剂。我们阐明了UHRA对肝素的选择性及其无毒性质的机理基础。基于等温滴定量热法的具有不同甲氧基聚乙二醇(mPEG)刷结构的UHRA与UFH的结合研究是溶液条件(包括离子强度)的函数,揭示了mPEG链对静电结合施加熵的惩罚。结合研究证实,与鱼精蛋白或N-UHRA(无mPEG链的UHRA的截短类似物)不同,UHRA中的mPEG链避免了与血液蛋白的非特异性相互作用,并通过空间排斥和Donnan屏蔽作用相结合提供了对肝素的选择性(a平衡F el和F steric)。凝血分析表明,带有mPEG链的UHRA不会对凝血产生不利影响,并在很宽的浓度范围内中和了UFH。相反,N-UHRA和鱼精蛋白显示出固有的抗凝活性,并显示出窄的UFH中和浓度窗口。此外,正如原子力显微镜和动态光散射研究所揭示的,我们发现mPEG链调节解毒剂-UFH复合物的大小。与N-UHRA和鱼精蛋白相比,具有mPEG链的UHRA分子与UFH形成较小的复合物。最后,荧光和ELISA实验表明,UHRA会破坏抗凝血酶-UFH复合物,以中和肝素的活性。
更新日期:2017-09-19
中文翻译:

具有通用肝素逆转活性的聚合物疗法:分子设计和功能机制。
肝素被广泛用于预防手术期间的血液凝结和血栓形成的治疗。然而,与肝素治疗相关的出血是一个问题。鱼精蛋白是唯一批准的普通肝素(UFH)的解毒剂,可能引起不良心血管事件。在这里,我们描述了一种独特的分子设计,用于开发称为通用肝素逆转剂(UHRA)的合成树突状聚阳离子,这是所有临床使用的肝素抗凝剂的解毒剂。我们阐明了UHRA对肝素的选择性及其无毒性质的机理基础。基于等温滴定量热法的具有不同甲氧基聚乙二醇(mPEG)刷结构的UHRA与UFH的结合研究是溶液条件(包括离子强度)的函数,揭示了mPEG链对静电结合施加熵的惩罚。结合研究证实,与鱼精蛋白或N-UHRA(无mPEG链的UHRA的截短类似物)不同,UHRA中的mPEG链避免了与血液蛋白的非特异性相互作用,并通过空间排斥和Donnan屏蔽作用相结合提供了对肝素的选择性(a平衡F el和F steric)。凝血分析表明,带有mPEG链的UHRA不会对凝血产生不利影响,并在很宽的浓度范围内中和了UFH。相反,N-UHRA和鱼精蛋白显示出固有的抗凝活性,并显示出窄的UFH中和浓度窗口。此外,正如原子力显微镜和动态光散射研究所揭示的,我们发现mPEG链调节解毒剂-UFH复合物的大小。与N-UHRA和鱼精蛋白相比,具有mPEG链的UHRA分子与UFH形成较小的复合物。最后,荧光和ELISA实验表明,UHRA会破坏抗凝血酶-UFH复合物,以中和肝素的活性。

































 京公网安备 11010802027423号
京公网安备 11010802027423号