当前位置:
X-MOL 学术
›
J. Phys. Chem. C
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
DFT Analysis of NO Oxidation Intermediates on Undoped and Doped LaCoO3 Perovskite
The Journal of Physical Chemistry C ( IF 3.3 ) Pub Date : 2015-08-26 00:00:00 , DOI: 10.1021/acs.jpcc.5b06351 Michael W. Penninger 1 , Chang Hwan Kim 2 , Levi T. Thompson 3 , William F. Schneider 1
The Journal of Physical Chemistry C ( IF 3.3 ) Pub Date : 2015-08-26 00:00:00 , DOI: 10.1021/acs.jpcc.5b06351 Michael W. Penninger 1 , Chang Hwan Kim 2 , Levi T. Thompson 3 , William F. Schneider 1
Affiliation
|
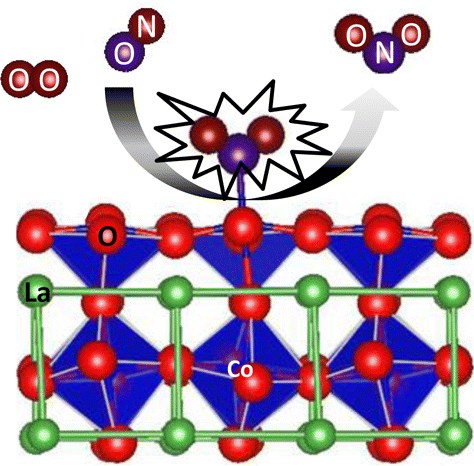
Perovskites are of interest as low-cost replacements for Pt-based NO oxidation catalysts. While the mechanism of Pt-catalyzed NO oxidation is fairly well understood, such is not the case for the oxides. The perovskite LaCoO3 itself has been shown to have good NO oxidation activity, and Sr substitution improves NO oxidation rates and reduces NO2 inhibition. In this work, we report density functional theory (DFT) results for the adsorption of NOx (x = 1, 2) to the undoped and Sr doped (100) LaO and CoO2 terminated LaCoO3. Further, we used first-principles thermodynamic models to determine the most common surface species under NO oxidation conditions. Nitrates and adsorbed NO are most stable on the LaO and CoO2 terminations, respectively. We explored the relative free energies of surface and vacancy-mediated pathways for NO oxidation. The vacancy-mediated pathways suffer from energetically costly removal of surface oxygen, while the surface pathway is most feasible for NO oxidation to occur. The perovskite surface free energy reaction pathway is compared to RuO2, MgO, and Pt. The CoO2 termination surface pathway is energetically most similar to that of Pt and is considered to be the most plausible for NO oxidation.
中文翻译:

LaCoO 3和未掺杂LaCoO 3钙钛矿上NO氧化中间体的DFT分析
钙钛矿作为Pt基NO氧化催化剂的低成本替代品而受到关注。尽管相当清楚地了解了Pt催化的NO氧化的机理,但对于氧化物却并非如此。钙钛矿型LaCoO 3本身已显示出良好的NO氧化活性,而Sr取代可提高NO的氧化速率并减少NO 2的抑制作用。在这项工作中,我们报告了密度泛函理论(DFT)的结果,表明NO x(x = 1,2)吸附到未掺杂和Sr掺杂(100)LaO和CoO 2终止的LaCoO 3。此外,我们使用第一性原理热力学模型来确定NO氧化条件下最常见的表面物种。硝酸盐和吸附的NO分别在LaO和CoO 2末端上最稳定。我们探索了表面和空位介导的NO氧化途径的相对自由能。空位介导的途径遭受了能量上昂贵的表面氧的去除,而表面途径对于发生NO氧化是最可行的。将钙钛矿表面自由能反应路径与RuO 2,MgO和Pt进行比较。CoO 2终止表面途径在能量上与Pt最相似,并且被认为是最合理的NO氧化途径。
更新日期:2015-08-26
中文翻译:

LaCoO 3和未掺杂LaCoO 3钙钛矿上NO氧化中间体的DFT分析
钙钛矿作为Pt基NO氧化催化剂的低成本替代品而受到关注。尽管相当清楚地了解了Pt催化的NO氧化的机理,但对于氧化物却并非如此。钙钛矿型LaCoO 3本身已显示出良好的NO氧化活性,而Sr取代可提高NO的氧化速率并减少NO 2的抑制作用。在这项工作中,我们报告了密度泛函理论(DFT)的结果,表明NO x(x = 1,2)吸附到未掺杂和Sr掺杂(100)LaO和CoO 2终止的LaCoO 3。此外,我们使用第一性原理热力学模型来确定NO氧化条件下最常见的表面物种。硝酸盐和吸附的NO分别在LaO和CoO 2末端上最稳定。我们探索了表面和空位介导的NO氧化途径的相对自由能。空位介导的途径遭受了能量上昂贵的表面氧的去除,而表面途径对于发生NO氧化是最可行的。将钙钛矿表面自由能反应路径与RuO 2,MgO和Pt进行比较。CoO 2终止表面途径在能量上与Pt最相似,并且被认为是最合理的NO氧化途径。

































 京公网安备 11010802027423号
京公网安备 11010802027423号