当前位置:
X-MOL 学术
›
J. Am. Chem. Soc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Proton-Coupled Electron Transfer in the Reduction of Arenes by SmI2–Water Complexes
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2015-08-26 , DOI: 10.1021/jacs.5b07518
Tesia V. Chciuk 1 , Robert A. Flowers 1
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2015-08-26 , DOI: 10.1021/jacs.5b07518
Tesia V. Chciuk 1 , Robert A. Flowers 1
Affiliation
|
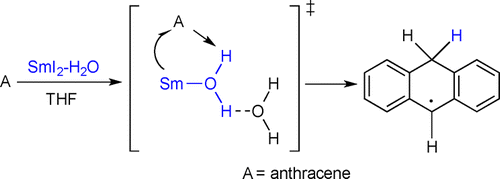
The presence of water has a significant impact on the reduction of substrates by SmI2. The reactivity of the Sm(II)-water reducing system and the relationship between sequential or concerted electron-transfer, proton-transfer is not well understood. In this work, we demonstrate that the reduction of an arene by SmI2-water proceeds through an initial proton-coupled electron transfer. The use of thermochemical data available in the literature shows that upon coordination of water to Sm(II) in THF, significant weakening of the O-H bond occurs. The derived value of nearly 73 kcal/mol for the decrease in the bond dissociation energy of the O-H bond in the Sm(II)-water complex is the largest reported to date for low-valent reductants containing bound water.
中文翻译:

SmI2-水配合物还原芳烃中的质子耦合电子转移
水的存在对 SmI2 对底物的还原有显着影响。Sm(II)-减水系统的反应性以及顺序或协同电子转移、质子转移之间的关系尚不清楚。在这项工作中,我们证明了 SmI2-水对芳烃的还原是通过初始质子耦合电子转移进行的。使用文献中可用的热化学数据表明,在 THF 中水与 Sm(II) 配位后,OH 键会显着减弱。Sm(II)-水复合物中OH键的键解离能降低近73 kcal/mol的衍生值是迄今为止报道的含有结合水的低价还原剂的最大值。
更新日期:2015-08-26
中文翻译:

SmI2-水配合物还原芳烃中的质子耦合电子转移
水的存在对 SmI2 对底物的还原有显着影响。Sm(II)-减水系统的反应性以及顺序或协同电子转移、质子转移之间的关系尚不清楚。在这项工作中,我们证明了 SmI2-水对芳烃的还原是通过初始质子耦合电子转移进行的。使用文献中可用的热化学数据表明,在 THF 中水与 Sm(II) 配位后,OH 键会显着减弱。Sm(II)-水复合物中OH键的键解离能降低近73 kcal/mol的衍生值是迄今为止报道的含有结合水的低价还原剂的最大值。

































 京公网安备 11010802027423号
京公网安备 11010802027423号