当前位置:
X-MOL 学术
›
Nat. Commun.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Ampk phosphorylation of Ulk1 is required for targeting of mitochondria to lysosomes in exercise-induced mitophagy.
Nature Communications ( IF 14.7 ) Pub Date : 2017-09-15 , DOI: 10.1038/s41467-017-00520-9 Rhianna C. Laker , Joshua C. Drake , Rebecca J. Wilson , Vitor A. Lira , Bevan M. Lewellen , Karen A. Ryall , Carleigh C. Fisher , Mei Zhang , Jeffrey J. Saucerman , Laurie J. Goodyear , Mondira Kundu , Zhen Yan
Nature Communications ( IF 14.7 ) Pub Date : 2017-09-15 , DOI: 10.1038/s41467-017-00520-9 Rhianna C. Laker , Joshua C. Drake , Rebecca J. Wilson , Vitor A. Lira , Bevan M. Lewellen , Karen A. Ryall , Carleigh C. Fisher , Mei Zhang , Jeffrey J. Saucerman , Laurie J. Goodyear , Mondira Kundu , Zhen Yan
|
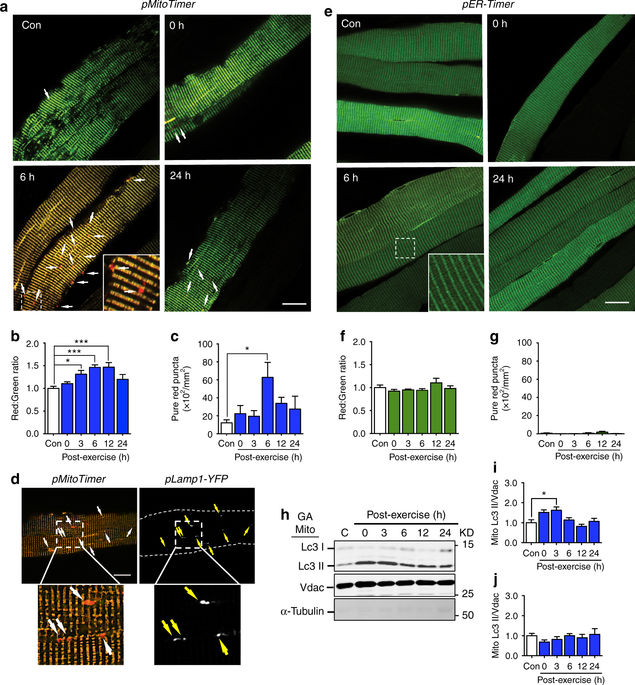
Mitochondrial health is critical for skeletal muscle function and is improved by exercise training through both mitochondrial biogenesis and removal of damaged/dysfunctional mitochondria via mitophagy. The mechanisms underlying exercise-induced mitophagy have not been fully elucidated. Here, we show that acute treadmill running in mice causes mitochondrial oxidative stress at 3-12 h and mitophagy at 6 h post-exercise in skeletal muscle. These changes were monitored using a novel fluorescent reporter gene, pMitoTimer, that allows assessment of mitochondrial oxidative stress and mitophagy in vivo, and were preceded by increased phosphorylation of AMP activated protein kinase (Ampk) at tyrosine 172 and of unc-51 like autophagy activating kinase 1 (Ulk1) at serine 555. Using mice expressing dominant negative and constitutively active Ampk in skeletal muscle, we demonstrate that Ulk1 activation is dependent on Ampk. Furthermore, exercise-induced metabolic adaptation requires Ulk1. These findings provide direct evidence of exercise-induced mitophagy and demonstrate the importance of Ampk-Ulk1 signaling in skeletal muscle.Exercise is associated with biogenesis and removal of dysfunctional mitochondria. Here the authors use a mitochondrial reporter gene to demonstrate the occurrence of mitophagy following exercise in mice, and show this is dependent on AMPK and ULK1 signaling.
中文翻译:

Ulk1的Ampk磷酸化是运动诱导的线粒体中将线粒体靶向溶酶体所必需的。
线粒体健康对骨骼肌功能至关重要,通过线粒体生物发生和通过线粒体清除受损/功能异常的线粒体的运动训练可以改善线粒体的健康。运动引起的线粒体吞噬的机制尚未完全阐明。在这里,我们显示在小鼠中运行的急性跑步机会在骨骼肌运动后3-12小时引起线粒体氧化应激,并在运动后6小时引起线粒体氧化。使用新的荧光报告基因pMitoTimer监测这些变化,该基因可以评估体内的线粒体氧化应激和线粒体吞噬,并在酪氨酸172处的AMP激活蛋白激酶(Ampk)和像自噬激活一样的unc-51磷酸化增加之前丝氨酸555的激酶1(Ulk1)。使用在骨骼肌中表达显性负性和组成性活性Ampk的小鼠,我们证明Ulk1激活依赖于Ampk。此外,运动引起的代谢适应需要Ulk1。这些发现提供了运动诱发的线粒体运动的直接证据,并证明了Ampk-Ulk1信号在骨骼肌中的重要性。运动与线粒体功能障碍的生物发生和清除相关。在这里,作者使用线粒体报告基因来证明小鼠运动后出现线粒体吞噬,并表明这依赖于AMPK和ULK1信号传导。这些发现提供了运动诱发的线粒体运动的直接证据,并证明了Ampk-Ulk1信号在骨骼肌中的重要性。运动与线粒体功能障碍的生物发生和清除相关。在这里,作者使用线粒体报告基因来证明小鼠运动后出现线粒体吞噬,并表明这依赖于AMPK和ULK1信号传导。这些发现提供了运动诱发的线粒体运动的直接证据,并证明了Ampk-Ulk1信号在骨骼肌中的重要性。运动与线粒体功能障碍的生物发生和清除相关。在这里,作者使用线粒体报告基因来证明小鼠运动后出现线粒体吞噬,并表明这依赖于AMPK和ULK1信号传导。
更新日期:2017-09-15
中文翻译:

Ulk1的Ampk磷酸化是运动诱导的线粒体中将线粒体靶向溶酶体所必需的。
线粒体健康对骨骼肌功能至关重要,通过线粒体生物发生和通过线粒体清除受损/功能异常的线粒体的运动训练可以改善线粒体的健康。运动引起的线粒体吞噬的机制尚未完全阐明。在这里,我们显示在小鼠中运行的急性跑步机会在骨骼肌运动后3-12小时引起线粒体氧化应激,并在运动后6小时引起线粒体氧化。使用新的荧光报告基因pMitoTimer监测这些变化,该基因可以评估体内的线粒体氧化应激和线粒体吞噬,并在酪氨酸172处的AMP激活蛋白激酶(Ampk)和像自噬激活一样的unc-51磷酸化增加之前丝氨酸555的激酶1(Ulk1)。使用在骨骼肌中表达显性负性和组成性活性Ampk的小鼠,我们证明Ulk1激活依赖于Ampk。此外,运动引起的代谢适应需要Ulk1。这些发现提供了运动诱发的线粒体运动的直接证据,并证明了Ampk-Ulk1信号在骨骼肌中的重要性。运动与线粒体功能障碍的生物发生和清除相关。在这里,作者使用线粒体报告基因来证明小鼠运动后出现线粒体吞噬,并表明这依赖于AMPK和ULK1信号传导。这些发现提供了运动诱发的线粒体运动的直接证据,并证明了Ampk-Ulk1信号在骨骼肌中的重要性。运动与线粒体功能障碍的生物发生和清除相关。在这里,作者使用线粒体报告基因来证明小鼠运动后出现线粒体吞噬,并表明这依赖于AMPK和ULK1信号传导。这些发现提供了运动诱发的线粒体运动的直接证据,并证明了Ampk-Ulk1信号在骨骼肌中的重要性。运动与线粒体功能障碍的生物发生和清除相关。在这里,作者使用线粒体报告基因来证明小鼠运动后出现线粒体吞噬,并表明这依赖于AMPK和ULK1信号传导。































 京公网安备 11010802027423号
京公网安备 11010802027423号