European Journal of Medicinal Chemistry ( IF 6.0 ) Pub Date : 2017-09-15 , DOI: 10.1016/j.ejmech.2017.09.018 Jiao Yang , Kai Chen , Guo Zhang , Qiu-Yuan Yang , Yue-Shan Li , Shen-Zhen Huang , Yan-Lin Wang , Wei Yang , Xiao-Juan Jiang , Heng-Xiu Yan , Jing-Qiang Zhu , Rong Xiang , You-Fu Luo , Wei-Min Li , Yu-Quan Wei , Lin-Li Li , Sheng-Yong Yang
|
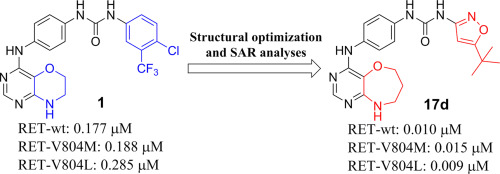
The RET tyrosine kinase is an important therapeutic target for medullary thyroid cancer (MTC), and drug resistance mutations of RET, particularly V804M and V804L, are a main challenge for the current targeted therapy of MTC based on RET inhibitors. In this investigation, we report the structural optimization and structure-activity relationship studies of N-phenyl-7,8-dihydro-6H-pyrimido[5,4-b][1,4]oxazin-4-amine derivatives as a new class of RET inhibitors. Among all the obtained kinase inhibitors, 1-(5-(tert-butyl)isoxazol-3-yl)-3-(4-((6,7,8,9-tetrahydropyrimido[5,4-b][1,4]oxazepin-4-yl)amino)phenyl)urea (17d) is a multi-kinase inhibitor and potently inhibits RET and its drug resistance mutants. It showed IC50 (half maximal inhibitory concentration) values of 0.010 μM, 0.015 μM, and 0.009 μM against RET-wild-type, RET-V804M, and RET-V804L, respectively. 17d displayed significant anti-viability potencies against various RET-driving tumor cell lines. In a xenograft mouse model of NIH3T3-RET-C634Y, 17d exhibited potent in vivo anti-tumor activity, and no obvious toxicity was observed. Mechanisms of action were also investigated by Western blot and immunohistochemical assays. Collectively, 17d could be a promising agent for the treatment of MTC, hence deserving a further investigation.
中文翻译:

N-苯基-7,8-二氢-6 H-嘧啶基[5,4-b] [1,4]恶嗪-4-胺衍生物作为新型RET抑制剂的结构优化和构效关系研究其耐药突变体
RET酪氨酸激酶是甲状腺髓样癌(MTC)的重要治疗靶标,RET的耐药性突变,特别是V804M和V804L,是目前基于RET抑制剂的MTC靶向治疗的主要挑战。在这项调查中,我们报告了N-苯基-7,8-二氢-6 H-嘧啶基[5,4-b] [1,4]恶嗪-4-胺衍生物的结构优化和构效关系研究。新型的RET抑制剂。在所有获得的激酶抑制剂中,1-(5-(叔丁基)异恶唑-3-基)-3-(4-((6,7,8,9-四氢嘧啶[5,4-b] [1, 4]氧杂氮杂-4-基)氨基)苯基)脲(17d)是一种多激酶抑制剂,可有效抑制RET及其耐药突变体。显示IC 50RET野生型,RET-V804M和RET-V804L的(最大半抑制浓度)值分别为0.010μM,0.015μM和0.009μM。图17d显示了针对各种RET驱动肿瘤细胞系的显着抗生存力。在NIH3T3-RET-C634Y的异种移植小鼠模型中,17d表现出有效的体内抗肿瘤活性,并且未观察到明显的毒性。还通过蛋白质印迹和免疫组织化学测定法研究了作用机理。总的来说,17d可能是治疗MTC的有前途的药物,因此值得进一步研究。


















































 京公网安备 11010802027423号
京公网安备 11010802027423号