当前位置:
X-MOL 学术
›
Org. Process Res. Dev.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Potential Safety Hazards Associated with Using Acetonitrile and a Strong Aqueous Base
Organic Process Research & Development ( IF 3.1 ) Pub Date : 2017-09-14 00:00:00 , DOI: 10.1021/acs.oprd.7b00158 Zhe Wang 1 , Steven M. Richter 1 , Michael J. Rozema 1 , Adam Schellinger 1 , Kimberly Smith 1 , José G. Napolitano 1
Organic Process Research & Development ( IF 3.1 ) Pub Date : 2017-09-14 00:00:00 , DOI: 10.1021/acs.oprd.7b00158 Zhe Wang 1 , Steven M. Richter 1 , Michael J. Rozema 1 , Adam Schellinger 1 , Kimberly Smith 1 , José G. Napolitano 1
Affiliation
|
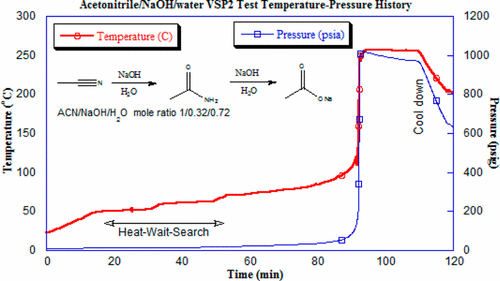
Acetonitrile, a common solvent in organic synthesis, can be hydrolyzed in the presence of a strong aqueous base, such as NaOH or KOH, which can propagate into a runaway reaction. For a process recently reviewed in our laboratory, a possible loss of cooling incident during the desired reaction was found to have the potential to self-heat to the onset temperature of this hydrolysis reaction. This base-catalyzed acetonitrile hydrolysis was determined to potentially escalate into a runaway reaction, where it could lead to secondary exothermic runaway reactions of the reaction mixture. The use of acetonitrile was preferred for the initial cyclization step. Thus, adequate removal of acetonitrile prior to the following step avoided the process safety hazard posed by the hydrolysis of the acetonitrile solvent. The findings presented in this work serve as an alert to chemists and engineers for the potential safety hazards when scaling up a process in which the solvent becomes a reactant.
中文翻译:

使用乙腈和强碱水溶液可能引起的安全隐患
乙腈是有机合成中的常用溶剂,可以在强碱水溶液(如NaOH或KOH)的存在下进行水解,该碱可以传播到失控反应中。对于最近在我们实验室中审查的方法,发现在所需反应过程中可能发生的冷却损失可能会自动加热至该水解反应的起始温度。经确定,这种碱催化的乙腈水解有可能升级为失控反应,这可能导致反应混合物发生二次放热失控反应。对于最初的环化步骤,乙腈的使用是优选的。因此,在随后的步骤之前充分除去乙腈避免了由乙腈溶剂的水解所引起的过程安全隐患。
更新日期:2017-09-15
中文翻译:

使用乙腈和强碱水溶液可能引起的安全隐患
乙腈是有机合成中的常用溶剂,可以在强碱水溶液(如NaOH或KOH)的存在下进行水解,该碱可以传播到失控反应中。对于最近在我们实验室中审查的方法,发现在所需反应过程中可能发生的冷却损失可能会自动加热至该水解反应的起始温度。经确定,这种碱催化的乙腈水解有可能升级为失控反应,这可能导致反应混合物发生二次放热失控反应。对于最初的环化步骤,乙腈的使用是优选的。因此,在随后的步骤之前充分除去乙腈避免了由乙腈溶剂的水解所引起的过程安全隐患。


















































 京公网安备 11010802027423号
京公网安备 11010802027423号