当前位置:
X-MOL 学术
›
ACS Chem. Biol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
An Anion−π Interaction Strongly Stabilizes the β-Sheet Protein WW
ACS Chemical Biology ( IF 3.5 ) Pub Date : 2017-09-14 00:00:00 , DOI: 10.1021/acschembio.7b00768 Mason S. Smith 1 , Eliza E. K. Lawrence 1 , Wendy M. Billings 1 , Kimberlee S. Larsen 1 , Natalie A. Bécar 1 , Joshua L. Price 1
ACS Chemical Biology ( IF 3.5 ) Pub Date : 2017-09-14 00:00:00 , DOI: 10.1021/acschembio.7b00768 Mason S. Smith 1 , Eliza E. K. Lawrence 1 , Wendy M. Billings 1 , Kimberlee S. Larsen 1 , Natalie A. Bécar 1 , Joshua L. Price 1
Affiliation
|
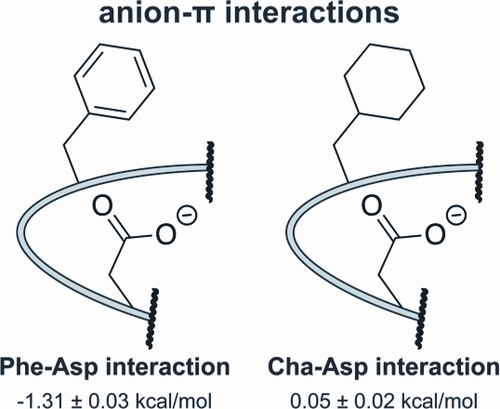
Anions have long been known to engage in stabilizing interactions with electron-deficient arenes. However, the precise nature and energetic contribution of anion−π interactions to protein stability remains a subject of debate. Here, we show that placing a negatively charged Asp in close proximity to electron-rich Phe in a reverse turn within the WW domain results in a favorable interaction that increases WW conformational stability by −1.3 kcal/mol.
中文翻译:

阴离子-π相互作用可强烈稳定β-Sheet蛋白WW
长期以来,人们一直认为阴离子会与缺乏电子的芳烃进行稳定的相互作用。然而,阴离子-π相互作用对蛋白质稳定性的确切性质和能量贡献仍然是一个争论的话题。在这里,我们表明,在WW域内反向放置带负电荷的Asp紧靠富电子的Phe会导致良好的相互作用,从而使WW构象稳定性增加-1.3 kcal / mol。
更新日期:2017-09-14
中文翻译:

阴离子-π相互作用可强烈稳定β-Sheet蛋白WW
长期以来,人们一直认为阴离子会与缺乏电子的芳烃进行稳定的相互作用。然而,阴离子-π相互作用对蛋白质稳定性的确切性质和能量贡献仍然是一个争论的话题。在这里,我们表明,在WW域内反向放置带负电荷的Asp紧靠富电子的Phe会导致良好的相互作用,从而使WW构象稳定性增加-1.3 kcal / mol。





























 京公网安备 11010802027423号
京公网安备 11010802027423号