Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Reproducible and scalable purification of extracellular vesicles using combined bind-elute and size exclusion chromatography.
Scientific Reports ( IF 3.8 ) Pub Date : 2017-Sep-14 , DOI: 10.1038/s41598-017-10646-x Giulia Corso , Imre Mäger , Yi Lee , André Görgens , Jarred Bultema , Bernd Giebel , Matthew J. A. Wood , Joel Z. Nordin , Samir EL Andaloussi
Scientific Reports ( IF 3.8 ) Pub Date : 2017-Sep-14 , DOI: 10.1038/s41598-017-10646-x Giulia Corso , Imre Mäger , Yi Lee , André Görgens , Jarred Bultema , Bernd Giebel , Matthew J. A. Wood , Joel Z. Nordin , Samir EL Andaloussi
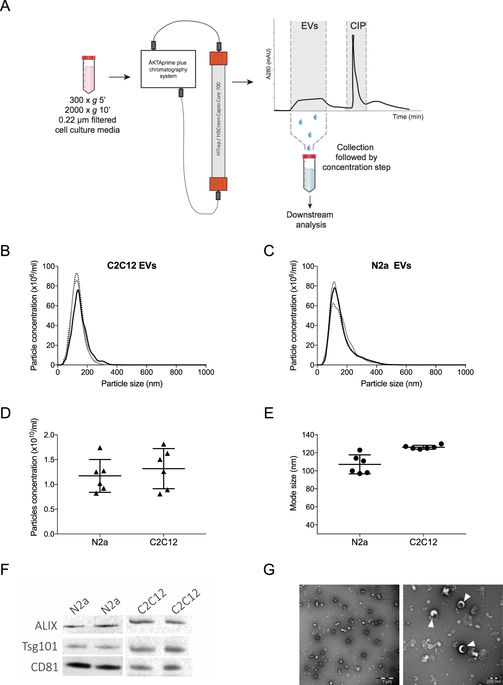
|
Extracellular vesicles (EVs) play a pivotal role in cell-to-cell communication and have been shown to take part in several physiological and pathological processes. EVs have traditionally been purified by ultracentrifugation (UC), however UC has limitations, including resulting in, operator-dependant yields, EV aggregation and altered EV morphology, and moreover is time consuming. Here we show that commercially available bind-elute size exclusion chromatography (BE-SEC) columns purify EVs with high yield (recovery ~ 80%) in a time-efficient manner compared to current methodologies. This technique is reproducible and scalable, and surface marker analysis by bead-based flow cytometry revealed highly similar expression signatures compared with UC-purified samples. Furthermore, uptake of eGFP labelled EVs in recipient cells was comparable between BE-SEC and UC samples. Hence, the BE-SEC based EV purification method represents an important methodological advance likely to facilitate robust and reproducible studies of EV biology and therapeutic application.
中文翻译:

使用结合洗脱和大小排阻色谱法可重现和可扩展地纯化细胞外囊泡。
细胞外囊泡(EVs)在细胞间通讯中起着关键作用,并已显示出参与多种生理和病理学过程的作用。电动汽车传统上是通过超速离心(UC)纯化的,但是UC具有局限性,包括导致依赖于操作员的产量,电动汽车的聚集和电动汽车形态的改变,而且非常耗时。在这里,我们显示,与当前方法相比,市售的结合洗脱体积排阻色谱法(BE-SEC)色谱柱能够以省时的方式以高效率(回收率〜80%)纯化EV。该技术具有可重现性和可扩展性,与基于UC纯化的样品相比,基于微珠的流式细胞仪进行的表面标记分析显示出高度相似的表达特征。此外,在BE-SEC和UC样品之间,受体细胞中eGFP标记的EV的摄取相当。因此,基于BE-SEC的EV纯化方法代表了重要的方法学进展,可能有助于对EV生物学和治疗应用进行可靠且可重复的研究。
更新日期:2017-09-14
中文翻译:

使用结合洗脱和大小排阻色谱法可重现和可扩展地纯化细胞外囊泡。
细胞外囊泡(EVs)在细胞间通讯中起着关键作用,并已显示出参与多种生理和病理学过程的作用。电动汽车传统上是通过超速离心(UC)纯化的,但是UC具有局限性,包括导致依赖于操作员的产量,电动汽车的聚集和电动汽车形态的改变,而且非常耗时。在这里,我们显示,与当前方法相比,市售的结合洗脱体积排阻色谱法(BE-SEC)色谱柱能够以省时的方式以高效率(回收率〜80%)纯化EV。该技术具有可重现性和可扩展性,与基于UC纯化的样品相比,基于微珠的流式细胞仪进行的表面标记分析显示出高度相似的表达特征。此外,在BE-SEC和UC样品之间,受体细胞中eGFP标记的EV的摄取相当。因此,基于BE-SEC的EV纯化方法代表了重要的方法学进展,可能有助于对EV生物学和治疗应用进行可靠且可重复的研究。


















































 京公网安备 11010802027423号
京公网安备 11010802027423号