当前位置:
X-MOL 学术
›
Asian J. Org. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Push–Pull Distyryl Boron Dipyrromethenes as Near‐Infrared Sensitizers for Dye‐Sensitized Solar Cells
Asian Journal of Organic Chemistry ( IF 2.8 ) Pub Date : 2017-08-09 , DOI: 10.1002/ajoc.201700282 Wen-Jing Shi 1, 2 , Takumi Kinoshita 3 , Dennis K. P. Ng 1
Asian Journal of Organic Chemistry ( IF 2.8 ) Pub Date : 2017-08-09 , DOI: 10.1002/ajoc.201700282 Wen-Jing Shi 1, 2 , Takumi Kinoshita 3 , Dennis K. P. Ng 1
Affiliation
|
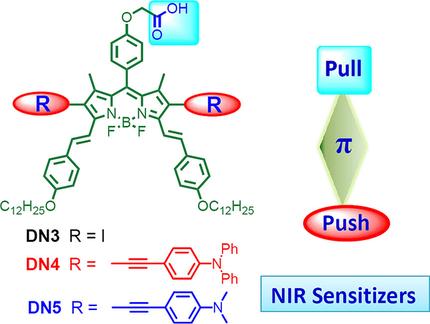
A diiodo distyryl boron dipyrromethene (BODIPY) with an ethyl ester group at the meso position was prepared. This compound underwent palladium‐catalyzed Sonogashira cross‐coupling reactions with 4‐(diphenylamino)phenylethyne and 4‐(dimethylamino)phenylethyne to give the corresponding dialkynyl derivatives. After alkaline hydrolysis and acidic work‐up, all three compounds were converted to the carboxyl analogues, which could bind to the surface of TiO2 for application in dye‐sensitized solar cells (DSSCs). These compounds, in particular the dialkynyl analogues, possessed a push–pull structure and a highly conjugated π system, which shifted the Q band to the near‐infrared region. Electrochemical studies revealed that they had appropriate HOMO and LUMO levels for the construction of DSSCs comprising a TiO2 electrode and an electrolyte containing an I3−/I− redox couple. The optimized structures, frontier molecular orbitals, and absorption spectra of these sensitizers were calculated using density functional theory. The diiodo distyryl BODIPY‐based DSSC showed a photovoltaic conversion efficiency of 2.78 % under AM 1.5G standard sunlight and a maximum incident photon‐to‐current conversion efficiency (IPCE) of >45 % at 780 nm. The DSSCs fabricated with the dialkynyl distyryl BODIPYs could achieve an IPCE value of approximately 20 % at 840 nm and a broader photoresponse over the whole visible range extending up to approximately 950 nm in the near‐infrared region.
中文翻译:

推挽式Distyryl硼双吡咯烷酮作为染料敏化太阳能电池的近红外增敏剂
制备了在内消旋位置具有乙基酯基的二碘二苯乙烯基硼二吡咯亚甲基(BODIPY)。该化合物与4-(二苯基氨基)苯乙炔和4-(二甲基氨基)苯乙炔进行钯催化的Sonogashira交叉偶联反应,得到相应的二炔基衍生物。经过碱水解和酸处理后,所有三种化合物都转化为羧基类似物,可以与TiO 2的表面结合用于染料敏化太阳能电池(DSSC)。这些化合物,特别是二炔基类似物,具有推挽结构和高度共轭的π系统,从而将Q波段移至近红外区域。电化学研究表明,他们有适当HOMO和LUMO能级为包含二氧化钛的DSSC的结构2电极和含有我的电解质3 - / I -氧化还原夫妇。使用密度泛函理论计算了这些敏化剂的优化结构,前沿分子轨道和吸收光谱。基于二碘二苯乙烯基BODIPY的DSSC在AM 1.5G标准日光下显示出2.78%的光电转换效率,在780 nm处的最大入射光子-电流转换效率(IPCE)> 45%。用二炔基二苯乙烯基BODIPYs制备的DSSC在840 nm处可实现约20%的IPCE值,并且在整个可见光范围内具有更宽的光响应,在近红外区域可扩展至约950 nm。
更新日期:2017-08-09
中文翻译:

推挽式Distyryl硼双吡咯烷酮作为染料敏化太阳能电池的近红外增敏剂
制备了在内消旋位置具有乙基酯基的二碘二苯乙烯基硼二吡咯亚甲基(BODIPY)。该化合物与4-(二苯基氨基)苯乙炔和4-(二甲基氨基)苯乙炔进行钯催化的Sonogashira交叉偶联反应,得到相应的二炔基衍生物。经过碱水解和酸处理后,所有三种化合物都转化为羧基类似物,可以与TiO 2的表面结合用于染料敏化太阳能电池(DSSC)。这些化合物,特别是二炔基类似物,具有推挽结构和高度共轭的π系统,从而将Q波段移至近红外区域。电化学研究表明,他们有适当HOMO和LUMO能级为包含二氧化钛的DSSC的结构2电极和含有我的电解质3 - / I -氧化还原夫妇。使用密度泛函理论计算了这些敏化剂的优化结构,前沿分子轨道和吸收光谱。基于二碘二苯乙烯基BODIPY的DSSC在AM 1.5G标准日光下显示出2.78%的光电转换效率,在780 nm处的最大入射光子-电流转换效率(IPCE)> 45%。用二炔基二苯乙烯基BODIPYs制备的DSSC在840 nm处可实现约20%的IPCE值,并且在整个可见光范围内具有更宽的光响应,在近红外区域可扩展至约950 nm。


















































 京公网安备 11010802027423号
京公网安备 11010802027423号