Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Butyrate-producing bacteria supplemented in vitro to Crohn's disease patient microbiota increased butyrate production and enhanced intestinal epithelial barrier integrity.
Scientific Reports ( IF 3.8 ) Pub Date : 2017-Sep-13 , DOI: 10.1038/s41598-017-11734-8 Annelies Geirnaert , Marta Calatayud , Charlotte Grootaert , Debby Laukens , Sarah Devriese , Guy Smagghe , Martine De Vos , Nico Boon , Tom Van de Wiele
Scientific Reports ( IF 3.8 ) Pub Date : 2017-Sep-13 , DOI: 10.1038/s41598-017-11734-8 Annelies Geirnaert , Marta Calatayud , Charlotte Grootaert , Debby Laukens , Sarah Devriese , Guy Smagghe , Martine De Vos , Nico Boon , Tom Van de Wiele
|
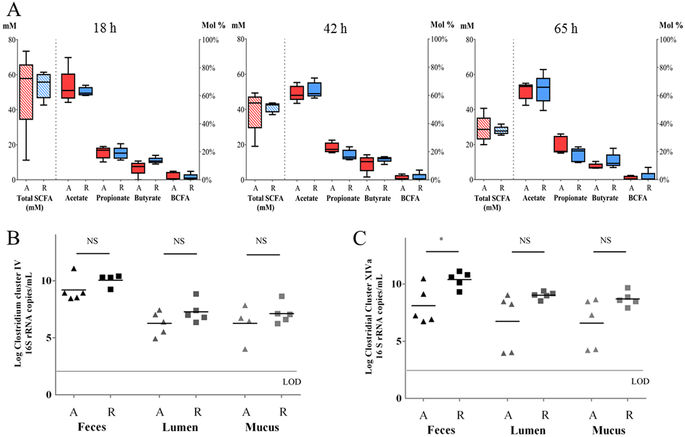
The management of the dysbiosed gut microbiota in inflammatory bowel diseases (IBD) is gaining more attention as a novel target to control this disease. Probiotic treatment with butyrate-producing bacteria has therapeutic potential since these bacteria are depleted in IBD patients and butyrate has beneficial effects on epithelial barrier function and overall gut health. However, studies assessing the effect of probiotic supplementation on microbe-microbe and host-microbe interactions are rare. In this study, butyrate-producing bacteria (three mono-species and one multispecies mix) were supplemented to the fecal microbial communities of ten Crohn's disease (CD) patients in an in vitro system simulating the mucus- and lumen-associated microbiota. Effects of supplementation in short-chain fatty acid levels, bacterial colonization of mucus environment and intestinal epithelial barrier function were evaluated. Treatment with F. prausnitzii and the mix of six butyrate-producers significantly increased the butyrate production by 5-11 mol%, and colonization capacity in mucus- and lumen-associated CD microbiota. Treatments with B. pullicaecorum 25-3T and the mix of six butyrate-producers improved epithelial barrier integrity in vitro. This study provides proof-of-concept data for the therapeutic potential of butyrate-producing bacteria in CD and supports the future preclinical development of a probiotic product containing butyrate-producing species.
中文翻译:

体外向克罗恩病患者微生物群补充的产生丁酸盐的细菌增加了丁酸盐的产生并增强了肠上皮屏障的完整性。
作为控制这种疾病的新靶标,炎症性肠病(IBD)中病原微生物肠道菌群的处理正受到越来越多的关注。用产生丁酸盐的细菌进行益生菌治疗具有治疗潜力,因为这些细菌在IBD患者中消耗are尽,而丁酸盐对上皮屏障功能和整体肠道健康具有有益作用。然而,评估益生菌补充剂对微生物-微生物和宿主-微生物相互作用的影响的研究很少。在这项研究中,在模拟粘液和管腔相关微生物的体外系统中,将产生丁酸盐的细菌(三种单物种和一种多物种的混合物)添加到了十名克罗恩病(CD)患者的粪便微生物群落中。补充对短链脂肪酸水平的影响,评价粘液环境的细菌定植和肠上皮屏障功能。用F. prausnitzii和六种丁酸盐生产者的混合物处理可显着提高丁酸盐产量5-11 mol%,并在与粘液和腔相关的CD微生物群中增加定殖能力。B. Pullicaecorum 25-3的治疗T和六种丁酸盐生产者的混合物在体外改善了上皮屏障的完整性。这项研究为CD中产生丁酸盐的细菌的治疗潜力提供了概念验证数据,并支持了含有丁酸盐产生物种的益生菌产品的未来临床前开发。
更新日期:2017-09-13
中文翻译:

体外向克罗恩病患者微生物群补充的产生丁酸盐的细菌增加了丁酸盐的产生并增强了肠上皮屏障的完整性。
作为控制这种疾病的新靶标,炎症性肠病(IBD)中病原微生物肠道菌群的处理正受到越来越多的关注。用产生丁酸盐的细菌进行益生菌治疗具有治疗潜力,因为这些细菌在IBD患者中消耗are尽,而丁酸盐对上皮屏障功能和整体肠道健康具有有益作用。然而,评估益生菌补充剂对微生物-微生物和宿主-微生物相互作用的影响的研究很少。在这项研究中,在模拟粘液和管腔相关微生物的体外系统中,将产生丁酸盐的细菌(三种单物种和一种多物种的混合物)添加到了十名克罗恩病(CD)患者的粪便微生物群落中。补充对短链脂肪酸水平的影响,评价粘液环境的细菌定植和肠上皮屏障功能。用F. prausnitzii和六种丁酸盐生产者的混合物处理可显着提高丁酸盐产量5-11 mol%,并在与粘液和腔相关的CD微生物群中增加定殖能力。B. Pullicaecorum 25-3的治疗T和六种丁酸盐生产者的混合物在体外改善了上皮屏障的完整性。这项研究为CD中产生丁酸盐的细菌的治疗潜力提供了概念验证数据,并支持了含有丁酸盐产生物种的益生菌产品的未来临床前开发。


















































 京公网安备 11010802027423号
京公网安备 11010802027423号