当前位置:
X-MOL 学术
›
J. Org. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Ph3P/I2-Mediated Synthesis of N,N′,N″-Substituted Guanidines and 2-Iminoimidazolin-4-ones from Aryl Isothiocyanates
The Journal of Organic Chemistry ( IF 3.3 ) Pub Date : 2017-09-12 00:00:00 , DOI: 10.1021/acs.joc.7b01794
Sirilak Wangngae 1 , Mookda Pattarawarapan 1 , Wong Phakhodee 1
The Journal of Organic Chemistry ( IF 3.3 ) Pub Date : 2017-09-12 00:00:00 , DOI: 10.1021/acs.joc.7b01794
Sirilak Wangngae 1 , Mookda Pattarawarapan 1 , Wong Phakhodee 1
Affiliation
|
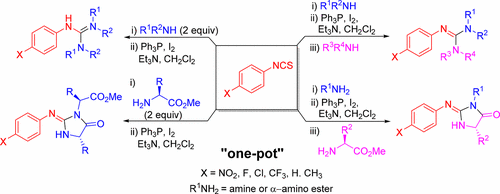
A convenient one-pot procedure for the synthesis of acyclic and cyclic guanidines mediated by the Ph3P/I2 system is described. Sequential condensation of aryl isothiocyanates with amines followed by dehydrosulfurization and guanylation could lead to both symmetric and unsymmetric N,N′,N″-substituted derivatives. Through a tandem guanylation–cyclization, a series of 2-iminoimidazolin-4-ones could also be prepared in good yields from the reaction of aryl isothiocyanates with amino acid methyl esters.
中文翻译:

pH值3 P / I 2的介导的合成N,N- ' ,N “ -取代的胍和2- Iminoimidazolin -4-酮从芳基异硫氰酸酯
描述了一种方便的一锅法,用于合成由Ph 3 P / I 2系统介导的无环和环状胍。芳基异硫氰酸酯与胺的顺序缩合,然后进行脱氢硫化和鸟苷化,可能同时导致对称和不对称的N,N ' ,N '' -取代的衍生物。通过串联的胍基化-环化反应,还可以从芳基异硫氰酸酯与氨基酸甲酯的反应中以高收率制备一系列2-iminoimidazolin-4-ones。
更新日期:2017-09-12
中文翻译:

pH值3 P / I 2的介导的合成N,N- ' ,N “ -取代的胍和2- Iminoimidazolin -4-酮从芳基异硫氰酸酯
描述了一种方便的一锅法,用于合成由Ph 3 P / I 2系统介导的无环和环状胍。芳基异硫氰酸酯与胺的顺序缩合,然后进行脱氢硫化和鸟苷化,可能同时导致对称和不对称的N,N ' ,N '' -取代的衍生物。通过串联的胍基化-环化反应,还可以从芳基异硫氰酸酯与氨基酸甲酯的反应中以高收率制备一系列2-iminoimidazolin-4-ones。

































 京公网安备 11010802027423号
京公网安备 11010802027423号