当前位置:
X-MOL 学术
›
J. Agric. Food Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Simultaneous Enantioselective Determination of the Chiral Fungicide Prothioconazole and Its Major Chiral Metabolite Prothioconazole-Desthio in Food and Environmental Samples by Ultraperformance Liquid Chromatography–Tandem Mass Spectrometry
Journal of Agricultural and Food Chemistry ( IF 5.7 ) Pub Date : 2017-09-11 00:00:00 , DOI: 10.1021/acs.jafc.7b02903 Zhaoxian Zhang 1 , Qing Zhang 1 , Beibei Gao 1 , Gaozhang Gou 2 , Lianshan Li 1 , Haiyan Shi 1 , Minghua Wang 1
Journal of Agricultural and Food Chemistry ( IF 5.7 ) Pub Date : 2017-09-11 00:00:00 , DOI: 10.1021/acs.jafc.7b02903 Zhaoxian Zhang 1 , Qing Zhang 1 , Beibei Gao 1 , Gaozhang Gou 2 , Lianshan Li 1 , Haiyan Shi 1 , Minghua Wang 1
Affiliation
|
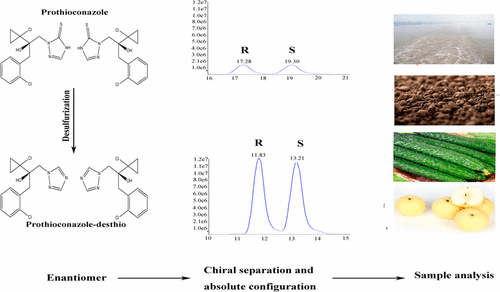
An efficient and sensitive chiral analytical method was established for the determination of the chiral fungicide prothioconazole and its major chiral metabolite prothioconazole-desthio in agricultural and environmental samples using ultraperformance liquid chromatography–tandem mass spectrometry. The optical rotation and absolute configuration of enantiomers were identified by optical rotation detector and electronic circular dichroism spectra. The elution order of prothioconazole and its chiral metabolite enantiomers was R-(+)-prothioconazole-desthio, S-(−)-prothioconazole-desthio, R-(−)-prothioconazole, and S-(+)-prothioconazole. The mean recoveries from the samples was 71.8–102.0% with intraday relative standard deviations (RSDs) of 0.3–11.9% and interday RSDs of 0.9–10.6%. The formation of prothioconazole-desthio was studied in soil under field conditions and enantioselective degradation was observed for chiral prothioconazole. Remarkable enantioselective degradation was observed: R-prothioconazole degraded preferentially with EF values from 0.48 to 0.37. Although prothioconazole-desthio is the most remarkably bioactive metabolite, no obvious enantioselective behavior was observed in soil. These results may help to systematically evaluate prothioconazole and its metabolites in food and environmental safety.
中文翻译:

超高效液相色谱-串联质谱法同时测定食品和环境样品中的手性杀菌剂丙硫康唑及其主要手性代谢物丙硫康-去硫
建立了一种高效,灵敏的手性分析方法,采用超高效液相色谱-串联质谱法测定农业和环境样品中的手性杀菌剂普罗康康唑及其主要手性代谢物普罗康康唑-去硫基。对映体的旋光性和绝对构型通过旋光检测器和电子圆二色性光谱进行鉴定。Prothioconazole及其手性代谢物对映体的洗脱顺序为R -(+)-prothioconazole-desthio,S -(-)-prothioconazole-desthio,R -(-)- prothioconazole和S-(+)-原硫代康唑。样品的平均回收率为71.8-102.0%,日内相对标准偏差(RSD)为0.3-11.9%,日间RSD为0.9-10.6%。在田间条件下,在土壤中研究了原硫代康康唑-去硫基的形成,并观察到手性原硫代康康唑的对映选择性降解。观察到显着的对映选择性降解:R-原硫代康唑以EF值从0.48至0.37优先降解。尽管丙硫康唑-去硫是最显着的生物活性代谢物,但在土壤中未观察到明显的对映选择性行为。这些结果可能有助于系统评估食品和环境安全中的原硫代康唑及其代谢产物。
更新日期:2017-09-11
中文翻译:

超高效液相色谱-串联质谱法同时测定食品和环境样品中的手性杀菌剂丙硫康唑及其主要手性代谢物丙硫康-去硫
建立了一种高效,灵敏的手性分析方法,采用超高效液相色谱-串联质谱法测定农业和环境样品中的手性杀菌剂普罗康康唑及其主要手性代谢物普罗康康唑-去硫基。对映体的旋光性和绝对构型通过旋光检测器和电子圆二色性光谱进行鉴定。Prothioconazole及其手性代谢物对映体的洗脱顺序为R -(+)-prothioconazole-desthio,S -(-)-prothioconazole-desthio,R -(-)- prothioconazole和S-(+)-原硫代康唑。样品的平均回收率为71.8-102.0%,日内相对标准偏差(RSD)为0.3-11.9%,日间RSD为0.9-10.6%。在田间条件下,在土壤中研究了原硫代康康唑-去硫基的形成,并观察到手性原硫代康康唑的对映选择性降解。观察到显着的对映选择性降解:R-原硫代康唑以EF值从0.48至0.37优先降解。尽管丙硫康唑-去硫是最显着的生物活性代谢物,但在土壤中未观察到明显的对映选择性行为。这些结果可能有助于系统评估食品和环境安全中的原硫代康唑及其代谢产物。

































 京公网安备 11010802027423号
京公网安备 11010802027423号