当前位置:
X-MOL 学术
›
J. Phys. Chem. C
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Ab Initio Study of Stability of Na2Fe2(SO4)3, a High Potential Na-Ion Battery Cathode Material
The Journal of Physical Chemistry C ( IF 3.3 ) Pub Date : 2017-09-08 00:00:00 , DOI: 10.1021/acs.jpcc.7b02479 Maxim Shishkin 1 , Hirofumi Sato 1, 2
The Journal of Physical Chemistry C ( IF 3.3 ) Pub Date : 2017-09-08 00:00:00 , DOI: 10.1021/acs.jpcc.7b02479 Maxim Shishkin 1 , Hirofumi Sato 1, 2
Affiliation
|
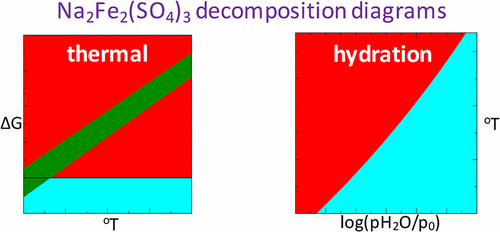
The free energies of thermal decomposition and hydration of Na2Fe2(SO4)3, used as a high operating potential Na-ion battery cathode material (3.8 V), are calculated with the help of the DFT+U method, augmented by magnetic exchange term. In addition, the energetics of hydration of low potential Na2Fe(SO4)·2H2O cathode (3.25 V) in humidified atmosphere is also analyzed. We find that DFT+U/magnetic exchange method can provide a reasonable agreement between calculated ground state properties of the studied materials (i.e., energetics and magnetic moments) and experiment. Using the evaluated total energies of solid materials and the free energies of the molecular species, we determined that Na2Fe2(SO4)3 is indeed thermally unstable at temperatures T ∼ 450 °C in agreement with experimental observations. Moreover, our calculations predict that Na2Fe2(SO4)3 can be hydrated in room temperature environment (T = 20 °C) also in a reasonable agreement with experiment. Overall, our work shows that the DFT+U method, augmented by magnetic exchange, can be used for analysis of thermal decomposition and hydration of complex compounds such as Na intercalated iron sulfates.
中文翻译:

从头开始研究高电位钠离子电池正极材料Na 2 Fe 2(SO 4)3的稳定性
借助DFT + U方法,通过磁增强,计算了用作高工作电势Na离子电池正极材料(3.8 V)的Na 2 Fe 2(SO 4)3的热分解和水合作用的自由能。交换期限。此外,还分析了低电位Na 2 Fe(SO 4)·2H 2 O阴极(3.25 V)在湿润气氛中的水合能。我们发现DFT + U磁交换法可以在所研究材料的计算基态性质(即高能和磁矩)与实验之间提供合理的一致性。使用固体材料和分子种类的自由能的评价总能量,我们确定的Na 2的Fe 2(SO 4)3确实热不稳定的温度下Ť〜450℃下,在与实验观察一致。此外,我们的计算预测Na 2 Fe 2(SO 4)3可以在室温环境下水合(T= 20°C),并且与实验合理吻合。总体而言,我们的工作表明,通过磁交换增强的DFT + U方法可用于分析复杂化合物(如Na插层式硫酸铁)的热分解和水合作用。
更新日期:2017-09-08
中文翻译:

从头开始研究高电位钠离子电池正极材料Na 2 Fe 2(SO 4)3的稳定性
借助DFT + U方法,通过磁增强,计算了用作高工作电势Na离子电池正极材料(3.8 V)的Na 2 Fe 2(SO 4)3的热分解和水合作用的自由能。交换期限。此外,还分析了低电位Na 2 Fe(SO 4)·2H 2 O阴极(3.25 V)在湿润气氛中的水合能。我们发现DFT + U磁交换法可以在所研究材料的计算基态性质(即高能和磁矩)与实验之间提供合理的一致性。使用固体材料和分子种类的自由能的评价总能量,我们确定的Na 2的Fe 2(SO 4)3确实热不稳定的温度下Ť〜450℃下,在与实验观察一致。此外,我们的计算预测Na 2 Fe 2(SO 4)3可以在室温环境下水合(T= 20°C),并且与实验合理吻合。总体而言,我们的工作表明,通过磁交换增强的DFT + U方法可用于分析复杂化合物(如Na插层式硫酸铁)的热分解和水合作用。





















































 京公网安备 11010802027423号
京公网安备 11010802027423号