当前位置:
X-MOL 学术
›
J. Phys. Chem. C
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Water Mediated Deactivation of Co3O4 Naonrods Catalyst for CO Oxidation and Resumption of Activity at and Above 373 K: Electronic Structural Aspects by NAPPES
The Journal of Physical Chemistry C ( IF 3.3 ) Pub Date : 2017-09-08 00:00:00 , DOI: 10.1021/acs.jpcc.7b05480
Ruchi Jain 1 , Kasala Prabhakar Reddy 1 , Manoj Kumar Ghosalya 1 , Chinnakonda S. Gopinath 1, 2
The Journal of Physical Chemistry C ( IF 3.3 ) Pub Date : 2017-09-08 00:00:00 , DOI: 10.1021/acs.jpcc.7b05480
Ruchi Jain 1 , Kasala Prabhakar Reddy 1 , Manoj Kumar Ghosalya 1 , Chinnakonda S. Gopinath 1, 2
Affiliation
|
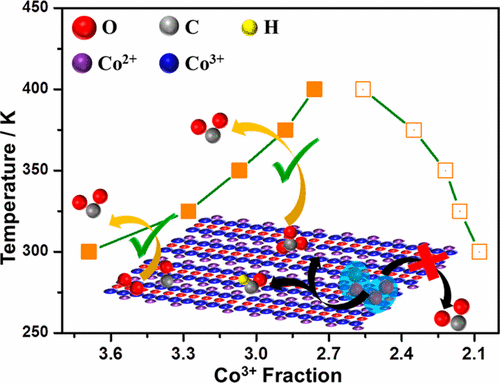
The catalytic activity of the Co3O4 nanorods (NRs) for the CO oxidation reaction and the effect of water on the catalytic reaction have been explored with near-ambient pressure photoelectron spectroscopy (NAPPES) and mass spectral analysis. Comparative NAPPES studies have been employed to understand the elucidation of the catalytic reaction pathway and the evolution of various surface species. The results confirm the suppression of the CO oxidation activity on the Co3O4 NRs in the presence of water vapor. Various type of surface species, such as CO(ads), hydroxyl, carbonate, formate, are found to be present on the catalyst surface depending on the reaction conditions. Vibrational features of CO, O2, and CO2 were observed and shift in binding energy of these features under the reaction conditions directly suggests a change in work function of the catalyst surface. Under dry conditions, CO couples with labile O atoms to form CO2; however, under wet conditions, CO predominantly interacts with surface OH groups resulting in the formation of carbonate and formate intermediates. In situ studies of oxidation of CO on Co3O4 shows that CO oxidation depends not only on surface Co3+ concentration but also influenced by Co3+/Co2+ ratio on the catalyst surface. The carbonate was found to be a reaction inhibitor at room temperature; however, it acts as an active intermediate at 375 K and above. Above the boiling point of water, Co3O4 NR surfaces begin to show the oxidation activity even in the presence of water vapor. The intrinsic role of intermediate species was used to derive a possible reaction mechanism under different reaction conditions.
中文翻译:

水介导的CO 3 O 4纳米棒催化剂的失活,用于CO氧化和在373 K及以上恢复活性:NAPPES的电子结构方面
通过近环境压力光电子能谱(NAPPES)和质谱分析,探索了Co 3 O 4纳米棒(NRs)对CO氧化反应的催化活性以及水对催化反应的影响。比较NAPPES研究已被用来理解催化反应途径的阐明和各种表面物种的演变。结果证实了在水蒸气存在下对Co 3 O 4 NRs上的CO氧化活性的抑制。根据反应条件,发现在催化剂表面上存在各种类型的表面物质,例如CO(ads),羟基,碳酸盐,甲酸盐。CO,O 2和CO的振动特征观察到2,并且在反应条件下这些特征的结合能的变化直接表明催化剂表面的功函数的变化。在干燥条件下,CO与不稳定的O原子偶合形成CO 2。然而,在潮湿条件下,CO主要与表面OH基团相互作用,导致形成碳酸盐和甲酸盐中间体。CO在Co 3 O 4上氧化的原位研究表明,CO氧化不仅取决于表面Co 3+的浓度,而且还受Co 3+ / Co 2+的影响。催化剂表面的比率。发现碳酸盐在室温下是一种反应抑制剂。但是,它在375 K及更高温度下充当活性中间体。高于水的沸点,即使在存在水蒸气的情况下,Co 3 O 4 NR表面也开始显示出氧化活性。中间体物种的内在作用被用来推导在不同反应条件下可能的反应机理。
更新日期:2017-09-08
中文翻译:

水介导的CO 3 O 4纳米棒催化剂的失活,用于CO氧化和在373 K及以上恢复活性:NAPPES的电子结构方面
通过近环境压力光电子能谱(NAPPES)和质谱分析,探索了Co 3 O 4纳米棒(NRs)对CO氧化反应的催化活性以及水对催化反应的影响。比较NAPPES研究已被用来理解催化反应途径的阐明和各种表面物种的演变。结果证实了在水蒸气存在下对Co 3 O 4 NRs上的CO氧化活性的抑制。根据反应条件,发现在催化剂表面上存在各种类型的表面物质,例如CO(ads),羟基,碳酸盐,甲酸盐。CO,O 2和CO的振动特征观察到2,并且在反应条件下这些特征的结合能的变化直接表明催化剂表面的功函数的变化。在干燥条件下,CO与不稳定的O原子偶合形成CO 2。然而,在潮湿条件下,CO主要与表面OH基团相互作用,导致形成碳酸盐和甲酸盐中间体。CO在Co 3 O 4上氧化的原位研究表明,CO氧化不仅取决于表面Co 3+的浓度,而且还受Co 3+ / Co 2+的影响。催化剂表面的比率。发现碳酸盐在室温下是一种反应抑制剂。但是,它在375 K及更高温度下充当活性中间体。高于水的沸点,即使在存在水蒸气的情况下,Co 3 O 4 NR表面也开始显示出氧化活性。中间体物种的内在作用被用来推导在不同反应条件下可能的反应机理。

































 京公网安备 11010802027423号
京公网安备 11010802027423号