当前位置:
X-MOL 学术
›
J. Agric. Food Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Transketolase A from E. coli Significantly Suppresses Protein Glycation by Glycolaldehyde and Glyoxal in Vitro
Journal of Agricultural and Food Chemistry ( IF 5.7 ) Pub Date : 2017-09-07 00:00:00 , DOI: 10.1021/acs.jafc.7b03183 Alexander Klaus 1 , Thorsten Pfirrmann 2 , Marcus A. Glomb 1
Journal of Agricultural and Food Chemistry ( IF 5.7 ) Pub Date : 2017-09-07 00:00:00 , DOI: 10.1021/acs.jafc.7b03183 Alexander Klaus 1 , Thorsten Pfirrmann 2 , Marcus A. Glomb 1
Affiliation
|
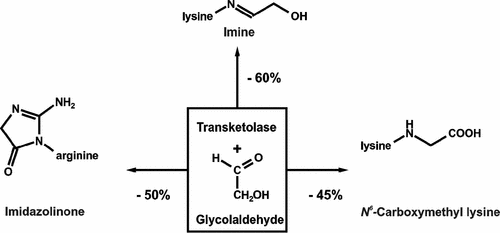
Short-chained carbonyl species such as glycolaldehyde and its oxidized pendant glyoxal are highly reactive Maillard agents, leading to the formation of protein modifications. These advanced glycation endproducts have gained considerable interest as they have been linked to various pathologies in vivo. The ability of transketolase to use glycolaldehyde as a substrate suggested the possibility to modulate carbonyl-driven Maillard reactions. Model incubations with recombinant transketolase A from Escherichia coli in the presence of bovine serum albumin and glycolaldehyde indeed led to a decrease in glycolaldehyde concentrations paralleled by the enzymatic conversion to erythrulose. As a result, reversibly protein-bound glycolaldehyde and the major final endproduct N6-carboxymethyl lysine were significantly reduced by approximately 50%, respectively. Glycolaldehyde is easily oxidized to glyoxal in the presence of amines and oxygen. In the presence of transketolase, the lower amounts of glycolaldehyde therefore also strongly suppressed the formation of glyoxal specific arginine modifications, measured as 5-(2-imino-5-oxo-1-imidazolidinyl)norvaline after acid hydrolysis.
中文翻译:

大肠杆菌的转酮醇酶A显着抑制糖醛和乙二醛在体外的蛋白质糖基化作用
短链羰基物种(例如乙醇醛)及其氧化的乙二醛侧基是高反应性美拉德试剂,导致形成蛋白质修饰。这些先进的糖基化终产物已经引起了人们的极大兴趣,因为它们已经与体内的各种病理联系在一起。转酮醇酶使用乙醇醛作为底物的能力表明有可能调节羰基驱动的美拉德反应。在牛血清白蛋白和乙醇醛存在下,用大肠杆菌的重组转酮醇酶A进行的模型温育确实导致了乙醇醛浓度的降低(与酶促转化为赤藓糖平行)。结果,可逆地结合蛋白质的乙醇醛和主要的最终产物N 6-羧甲基赖氨酸分别显着降低约50%。乙醇醛在胺和氧的存在下很容易被氧化成乙二醛。因此,在转酮醇酶的存在下,较低量的乙醇醛还强烈抑制了乙二醛特异精氨酸修饰的形成,该酸经酸水解后以5-(2-亚氨基-5-氧代-1-咪唑啉基)正缬氨酸测量。
更新日期:2017-09-07
中文翻译:

大肠杆菌的转酮醇酶A显着抑制糖醛和乙二醛在体外的蛋白质糖基化作用
短链羰基物种(例如乙醇醛)及其氧化的乙二醛侧基是高反应性美拉德试剂,导致形成蛋白质修饰。这些先进的糖基化终产物已经引起了人们的极大兴趣,因为它们已经与体内的各种病理联系在一起。转酮醇酶使用乙醇醛作为底物的能力表明有可能调节羰基驱动的美拉德反应。在牛血清白蛋白和乙醇醛存在下,用大肠杆菌的重组转酮醇酶A进行的模型温育确实导致了乙醇醛浓度的降低(与酶促转化为赤藓糖平行)。结果,可逆地结合蛋白质的乙醇醛和主要的最终产物N 6-羧甲基赖氨酸分别显着降低约50%。乙醇醛在胺和氧的存在下很容易被氧化成乙二醛。因此,在转酮醇酶的存在下,较低量的乙醇醛还强烈抑制了乙二醛特异精氨酸修饰的形成,该酸经酸水解后以5-(2-亚氨基-5-氧代-1-咪唑啉基)正缬氨酸测量。













































 京公网安备 11010802027423号
京公网安备 11010802027423号