当前位置:
X-MOL 学术
›
ACS Sustain. Chem. Eng.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Kinetic and Equilibrium Reactions of a New Heterocyclic Aqueous 4-Aminomethyltetrahydropyran (4-AMTHP) Absorbent for Post Combustion Carbon Dioxide (CO2) Capture Processes
ACS Sustainable Chemistry & Engineering ( IF 7.1 ) Pub Date : 2017-09-07 00:00:00 , DOI: 10.1021/acssuschemeng.7b02149 Lichun Li 1 , Sarah Clifford 1 , Graeme Puxty 2 , Marcel Maeder 1 , Robert Burns 1 , Hai Yu 2 , William Conway 2
ACS Sustainable Chemistry & Engineering ( IF 7.1 ) Pub Date : 2017-09-07 00:00:00 , DOI: 10.1021/acssuschemeng.7b02149 Lichun Li 1 , Sarah Clifford 1 , Graeme Puxty 2 , Marcel Maeder 1 , Robert Burns 1 , Hai Yu 2 , William Conway 2
Affiliation
|
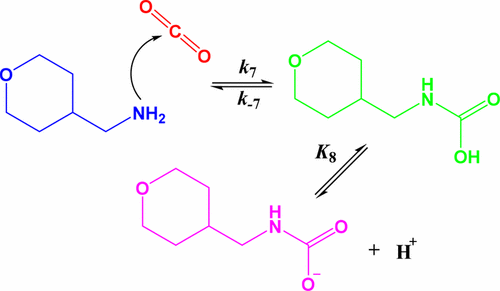
Aqueous amine absorbent processes remain at the forefront of existing technologies for the removal of CO2 from industrial and large-scale power generation flue gas streams. It is essential that improvements in amine-based absorbent technologies are made in order to reduce both capital and operational costs. Intimate understanding of the fundamental chemical behavior of new amine absorbent systems is an intelligent pathway toward higher efficiency amine-based CO2 capture processes. Herein, we investigate and report for the first time the complete temperature-dependent kinetic and equilibrium behavior of a new heterocyclic amine 4-aminomethyltetrahydropyran (4-AMTHP), with CO2, in aqueous solutions. Stopped-flow spectrophotometry, 1H NMR spectroscopy, and potentiometric titration measurements performed over the temperature range 25.0–45.0 °C and the corresponding rate constants for the reversible formation of the carbamic acid, together with equilibrium constants describing the stability of the carbamate, and the protonation of the amine are reported here. Thermodynamic analysis of the resulting constants using the Eyring, Arrhenius, and van’t Hoff relationships has revealed the activation energies, enthalpies, and entropies for the reactions, allowing a comparison to the industrial standard monoethanolamine (MEA). From the kinetic data, the performance of 4-AMTHP was found to be superior to MEA and in line with the established Brønsted relationship between the second-order rate constant and the protonation constant or basicity of the amine. The largely negative protonation enthalpy (−47 kJ/mol), among the key chemical drivers for CO2 regeneration, is again superior to MEA (−41 kJ/mol). Together, a combination of kinetic and equilibrium properties of 4-AMTHP strongly position 4-AMTHP as a promising candidate for more intensive evaluations as a CO2 capture absorbent.
中文翻译:

新型杂环4-氨基甲基四氢吡喃(4-AMTHP)吸收剂用于燃烧后二氧化碳(CO 2)捕集过程的动力学和平衡反应
胺吸收剂水处理方法仍然是从工业和大规模发电烟道气中去除CO 2的现有技术的最前沿。必须进行胺基吸收剂技术的改进,以减少资本和运营成本。对新型胺吸收剂系统基本化学行为的深入了解是迈向更高效率的基于胺的CO 2捕集工艺的明智途径。本文中,我们首次调查并报告了新型杂环胺4-氨基甲基四氢吡喃(4-AMTHP)与CO 2在水溶液中的完全温度依赖性的动力学和平衡行为。停止流式分光光度法,1在25.0–45.0°C的温度范围内进行的1 H NMR光谱和电位滴定测量以及可逆形成氨基甲酸的相应速率常数,以及描述氨基甲酸酯稳定性和胺质子化的平衡常数。在这里报道。使用Eyring,Arrhenius和van't Hoff关系式对所得常数进行热力学分析,发现反应的活化能,焓和熵,可以与工业标准单乙醇胺(MEA)进行比较。根据动力学数据,发现4-AMTHP的性能优于MEA,并且与胺的二级速率常数和质子化常数或碱度之间已建立的Brønsted关系相符。2再生再次优于MEA(-41 kJ / mol)。在一起,4-AMTHP的动力学和平衡特性的结合强烈地将4-AMTHP定位为有希望的候选者,可以作为CO 2捕获吸收剂进行更深入的评估。
更新日期:2017-09-07
中文翻译:

新型杂环4-氨基甲基四氢吡喃(4-AMTHP)吸收剂用于燃烧后二氧化碳(CO 2)捕集过程的动力学和平衡反应
胺吸收剂水处理方法仍然是从工业和大规模发电烟道气中去除CO 2的现有技术的最前沿。必须进行胺基吸收剂技术的改进,以减少资本和运营成本。对新型胺吸收剂系统基本化学行为的深入了解是迈向更高效率的基于胺的CO 2捕集工艺的明智途径。本文中,我们首次调查并报告了新型杂环胺4-氨基甲基四氢吡喃(4-AMTHP)与CO 2在水溶液中的完全温度依赖性的动力学和平衡行为。停止流式分光光度法,1在25.0–45.0°C的温度范围内进行的1 H NMR光谱和电位滴定测量以及可逆形成氨基甲酸的相应速率常数,以及描述氨基甲酸酯稳定性和胺质子化的平衡常数。在这里报道。使用Eyring,Arrhenius和van't Hoff关系式对所得常数进行热力学分析,发现反应的活化能,焓和熵,可以与工业标准单乙醇胺(MEA)进行比较。根据动力学数据,发现4-AMTHP的性能优于MEA,并且与胺的二级速率常数和质子化常数或碱度之间已建立的Brønsted关系相符。2再生再次优于MEA(-41 kJ / mol)。在一起,4-AMTHP的动力学和平衡特性的结合强烈地将4-AMTHP定位为有希望的候选者,可以作为CO 2捕获吸收剂进行更深入的评估。






























 京公网安备 11010802027423号
京公网安备 11010802027423号