当前位置:
X-MOL 学术
›
J. Am. Chem. Soc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Chloride Oxidation by Ruthenium Excited-States in Solution
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2017-09-07 00:00:00 , DOI: 10.1021/jacs.7b06762 Sara A. M. Wehlin 1 , Ludovic Troian-Gautier 1 , Guocan Li 1 , Gerald J. Meyer 1
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2017-09-07 00:00:00 , DOI: 10.1021/jacs.7b06762 Sara A. M. Wehlin 1 , Ludovic Troian-Gautier 1 , Guocan Li 1 , Gerald J. Meyer 1
Affiliation
|
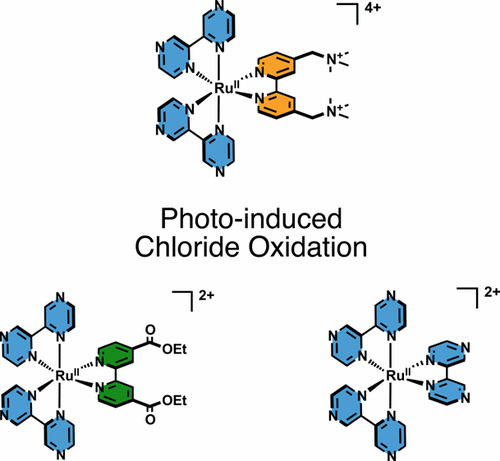
Photodriven HCl splitting to produce solar fuels is an important goal that requires strong photo-oxidants capable of chloride oxidation. In a molecular approach toward this goal, three ruthenium compounds with 2,2′-bipyrazine backbones were found to oxidize chloride ions in acetone solution. Nanosecond transient absorption measurements provide compelling evidence for excited-state electron transfer from chloride to the Ru metal center with rate constants in excess of 1010 M–1 s–1. The Cl atom product was trapped with an olefin. This reactivity was promoted through pre-organization of ground-state precursors in ion pairs. Chloride oxidation with a tetra-cationic ruthenium complex was most favorable, as the dicationic complexes were susceptible to photochemical ligand loss. Marcus analysis afforded an estimate of the chlorine formal reduction potential E°(Cl•/–) = 1.87 V vs NHE that is at least 300 meV more favorable than the accepted values in water.
中文翻译:

溶液中钌激发态对氯化物的氧化作用
光驱动的HCl分解产生太阳能燃料是一个重要的目标,需要能够氯化物氧化的强光氧化剂。在实现该目标的分子方法中,发现具有2,2'-联吡嗪骨架的三种钌化合物可氧化丙酮溶液中的氯离子。纳秒瞬态吸收测量提供了令人信服的证据,证明了从氯化物到Ru金属中心的激发态电子的传输速率常数超过10 10 M –1 s –1。Cl原子产物被烯烃捕获。这种反应性是通过离子对中基态前体的预组织来促进的。用四阳离子钌配合物进行氯化物氧化是最有利的,因为双金属配合物易受光化学配体的损失。Marcus分析提供了氯形式还原电位E °(Cl •/ –)= 1.87 V vs. NHE的估算值,该值至少比水中的可接受值高300 meV。
更新日期:2017-09-07
中文翻译:

溶液中钌激发态对氯化物的氧化作用
光驱动的HCl分解产生太阳能燃料是一个重要的目标,需要能够氯化物氧化的强光氧化剂。在实现该目标的分子方法中,发现具有2,2'-联吡嗪骨架的三种钌化合物可氧化丙酮溶液中的氯离子。纳秒瞬态吸收测量提供了令人信服的证据,证明了从氯化物到Ru金属中心的激发态电子的传输速率常数超过10 10 M –1 s –1。Cl原子产物被烯烃捕获。这种反应性是通过离子对中基态前体的预组织来促进的。用四阳离子钌配合物进行氯化物氧化是最有利的,因为双金属配合物易受光化学配体的损失。Marcus分析提供了氯形式还原电位E °(Cl •/ –)= 1.87 V vs. NHE的估算值,该值至少比水中的可接受值高300 meV。

































 京公网安备 11010802027423号
京公网安备 11010802027423号