当前位置:
X-MOL 学术
›
Nat. Commun.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Tau exacerbates excitotoxic brain damage in an animal model of stroke.
Nature Communications ( IF 14.7 ) Pub Date : 2017-09-07 , DOI: 10.1038/s41467-017-00618-0 Mian Bi , Amadeus Gladbach , Janet van Eersel , Arne Ittner , Magdalena Przybyla , Annika van Hummel , Sook Wern Chua , Julia van der Hoven , Wei S. Lee , Julius Müller , Jasneet Parmar , Georg von Jonquieres , Holly Stefen , Ernesto Guccione , Thomas Fath , Gary D. Housley , Matthias Klugmann , Yazi D. Ke , Lars M. Ittner
Nature Communications ( IF 14.7 ) Pub Date : 2017-09-07 , DOI: 10.1038/s41467-017-00618-0 Mian Bi , Amadeus Gladbach , Janet van Eersel , Arne Ittner , Magdalena Przybyla , Annika van Hummel , Sook Wern Chua , Julia van der Hoven , Wei S. Lee , Julius Müller , Jasneet Parmar , Georg von Jonquieres , Holly Stefen , Ernesto Guccione , Thomas Fath , Gary D. Housley , Matthias Klugmann , Yazi D. Ke , Lars M. Ittner
|
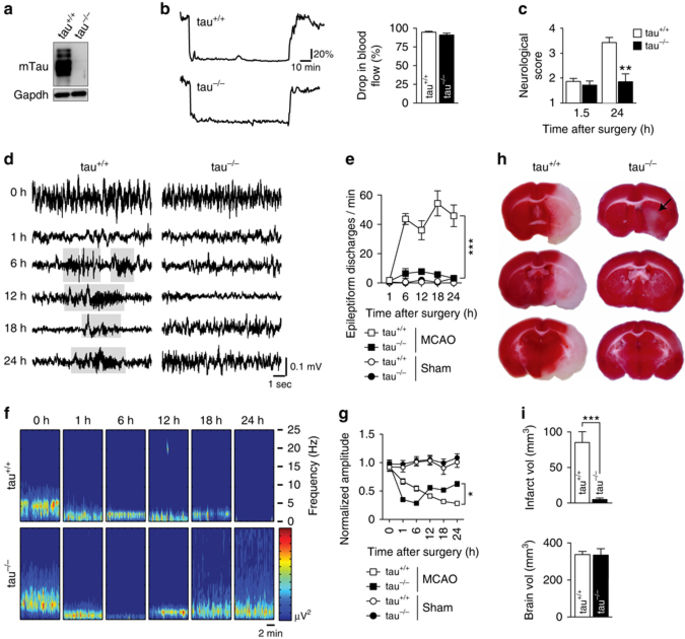
Neuronal excitotoxicity induced by aberrant excitation of glutamatergic receptors contributes to brain damage in stroke. Here we show that tau-deficient (tau-/-) mice are profoundly protected from excitotoxic brain damage and neurological deficits following experimental stroke, using a middle cerebral artery occlusion with reperfusion model. Mechanistically, we show that this protection is due to site-specific inhibition of glutamate-induced and Ras/ERK-mediated toxicity by accumulation of Ras-inhibiting SynGAP1, which resides in a post-synaptic complex with tau. Accordingly, reducing SynGAP1 levels in tau-/- mice abolished the protection from pharmacologically induced excitotoxicity and middle cerebral artery occlusion-induced brain damage. Conversely, over-expression of SynGAP1 prevented excitotoxic ERK activation in wild-type neurons. Our findings suggest that tau mediates excitotoxic Ras/ERK signaling by controlling post-synaptic compartmentalization of SynGAP1.Excitotoxicity contributes to neuronal injury following stroke. Here the authors show that tau promotes excitotoxicity by a post-synaptic mechanism, involving site-specific control of ERK activation, in a mouse model of stroke.
中文翻译:

Tau在中风的动物模型中加剧了兴奋性中毒性脑损伤。
由谷氨酸能受体的异常刺激引起的神经元兴奋性毒性导致中风的脑损伤。在这里,我们显示了tau缺陷(tau -/-)小鼠在使用实验性中风后,使用再灌注模型对大脑中动脉进行了阻断,可以免受兴奋性中毒性脑损伤和神经功能缺损的侵害。从机制上讲,我们表明这种保护是由于谷氨酸诱导的和Ras / ERK介导的毒性的位点特异性抑制,该抑制是通过Ras抑制的SynGAP1的积累而积累的,SynGAP1位于与tau的突触后复合物中。因此,降低tau中的SynGAP1水平-/-小鼠取消了对药理作用引起的兴奋性毒性和大脑中动脉闭塞引起的脑损伤的保护作用。相反,SynGAP1的过表达阻止了野生型神经元的兴奋性ERK活化。我们的发现表明tau通过控制SynGAP1的突触后区室化来介导兴奋性Ras / ERK信号传导。在这里,作者表明tau通过突触后机制(涉及中风的小鼠模型中对ERK激活的位点特异性控制)促进兴奋性毒性。
更新日期:2017-09-07
中文翻译:

Tau在中风的动物模型中加剧了兴奋性中毒性脑损伤。
由谷氨酸能受体的异常刺激引起的神经元兴奋性毒性导致中风的脑损伤。在这里,我们显示了tau缺陷(tau -/-)小鼠在使用实验性中风后,使用再灌注模型对大脑中动脉进行了阻断,可以免受兴奋性中毒性脑损伤和神经功能缺损的侵害。从机制上讲,我们表明这种保护是由于谷氨酸诱导的和Ras / ERK介导的毒性的位点特异性抑制,该抑制是通过Ras抑制的SynGAP1的积累而积累的,SynGAP1位于与tau的突触后复合物中。因此,降低tau中的SynGAP1水平-/-小鼠取消了对药理作用引起的兴奋性毒性和大脑中动脉闭塞引起的脑损伤的保护作用。相反,SynGAP1的过表达阻止了野生型神经元的兴奋性ERK活化。我们的发现表明tau通过控制SynGAP1的突触后区室化来介导兴奋性Ras / ERK信号传导。在这里,作者表明tau通过突触后机制(涉及中风的小鼠模型中对ERK激活的位点特异性控制)促进兴奋性毒性。





























 京公网安备 11010802027423号
京公网安备 11010802027423号