当前位置:
X-MOL 学术
›
ChemistrySelect
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Mechanistic Study on the Acylation ofBis(2,2,2‐Trifluoroethyl) Methylphosphonate by Carboxylic Esters
ChemistrySelect ( IF 1.9 ) Pub Date : 2017-09-05 , DOI: 10.1002/slct.201701161
Katalin Molnár 1 , László Takács 1 , Zoltán Mucsi 2 , Ferenc Faigl 3 , Zsuzsanna Kardos 1
ChemistrySelect ( IF 1.9 ) Pub Date : 2017-09-05 , DOI: 10.1002/slct.201701161
Katalin Molnár 1 , László Takács 1 , Zoltán Mucsi 2 , Ferenc Faigl 3 , Zsuzsanna Kardos 1
Affiliation
|
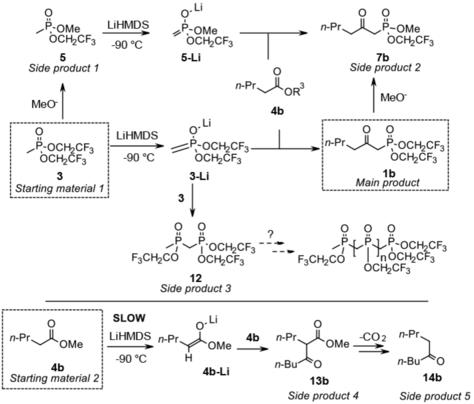
Application of bis(2,2,2‐trifluoroethyl) 2‐oxoalkylphosphonate reagents made the Horner‐Wadsworth‐Emmons reactions suitable for the formation of not only E‐ but Z‐unsaturated enones. By this modification, the synthesis of the geometric isomers can be performed by similar reactions. However, preparation of bis(2,2,2‐trifluoroethyl) phosphonates by the known methods, raises difficulties. To overcome this challenge, we have previously optimized the reaction parameters for the preparation of these phosphonates. During our work the main impurities were isolated and their structure elucidated. In the present work, we aimed at revealing the mechanisms of these sophisticated chemical transformations using computational methods and experimental results.
中文翻译:

羧酸酯对双(2,2,2-三氟乙基)甲基膦酸酯的酰化机理研究
双(2,2,2-三氟乙基)2-氧代烷基膦酸酯试剂的应用使Horner-Wadsworth-Emmons反应不仅适合形成E-而且还适合Z-不饱和的烯酮。通过这种修饰,可以通过类似的反应来合成几何异构体。然而,通过已知方法制备双(2,2,2-三氟乙基)膦酸酯会增加困难。为了克服这一挑战,我们先前已经优化了用于制备这些膦酸酯的反应参数。在我们的工作中,主要杂质被分离出来并阐明了其结构。在目前的工作中,我们旨在利用计算方法和实验结果揭示这些复杂的化学转化的机理。
更新日期:2017-09-05
中文翻译:

羧酸酯对双(2,2,2-三氟乙基)甲基膦酸酯的酰化机理研究
双(2,2,2-三氟乙基)2-氧代烷基膦酸酯试剂的应用使Horner-Wadsworth-Emmons反应不仅适合形成E-而且还适合Z-不饱和的烯酮。通过这种修饰,可以通过类似的反应来合成几何异构体。然而,通过已知方法制备双(2,2,2-三氟乙基)膦酸酯会增加困难。为了克服这一挑战,我们先前已经优化了用于制备这些膦酸酯的反应参数。在我们的工作中,主要杂质被分离出来并阐明了其结构。在目前的工作中,我们旨在利用计算方法和实验结果揭示这些复杂的化学转化的机理。

































 京公网安备 11010802027423号
京公网安备 11010802027423号