当前位置:
X-MOL 学术
›
Inorg. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Hemilability of the 1,2-Bis(dimethylphosphino)ethane (dmpe) Ligand in Cp*Mo(NO)(κ2-dmpe)
Inorganic Chemistry ( IF 4.3 ) Pub Date : 2017-09-06 00:00:00 , DOI: 10.1021/acs.inorgchem.7b01733 Aaron S. Holmes 1 , Brian O. Patrick 1 , Taleah M. Levesque 1 , Peter Legzdins 1
Inorganic Chemistry ( IF 4.3 ) Pub Date : 2017-09-06 00:00:00 , DOI: 10.1021/acs.inorgchem.7b01733 Aaron S. Holmes 1 , Brian O. Patrick 1 , Taleah M. Levesque 1 , Peter Legzdins 1
Affiliation
|
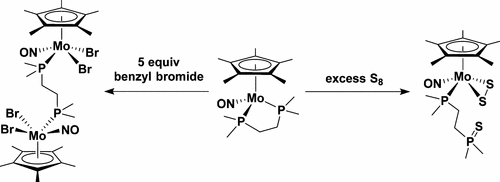
Reaction of Cp*Mo(NO)Cl2 with 1 equiv of 1,2-bis(dimethylphosphino)ethane (dmpe) in THF at ambient temperature forms [Cp*Mo(NO)(Cl)(κ2-dmpe)]Cl (1), which is isolable as an analytically pure yellow powder in 65% yield. Further addition of 2 equiv of Cp2Co to 1 in CH2Cl2 affords dark red Cp*Mo(NO)(κ2-dmpe) (2), which was isolated in 36% yield by recrystallization from Et2O at −30 °C. Reaction of a benzene solution of 2 with an equimolar amount of elemental sulfur results in the immediate production of dark blue (μ-S)[Cp*Mo(NO)(κ1-dmpeS)]2 (3), which is a rare example of a bimetallic transition-metal complex bridged by only a single sulfur atom and involving Mo═S═Mo bonding. In contrast, reaction of 2 with an excess of sulfur in benzene results in the formation of Cp*Mo(NO)(η2-S2)(κ1-dmpeS) (4). Complex 4 can also be formed by the addition of elemental sulfur to 3, thereby indicating that 3 is a precursor to 4. Cp*Mo(NO)(κ2-dmpe) (2) also undergoes interesting transformations when treated with organic bromides. For instance, reaction of 2 with 5 equiv benzyl bromide in THF produces the bimetallic complex (μ-dmpe)[Cp*Mo(NO)Br2]2 (5) and bibenzyl after 4 d at 70 °C probably via radical intermediates. In contrast to its reaction with benzyl bromide, complex 2 forms [Mo(NO)Br2(κ2-dmpe)]2 (6), olefin, alkane, and Cp*H when treated with 5 equiv of 1-bromopropane or 1-bromooctane in THF at 70 °C for 72 h. Interestingly, complex 2 does not display any reactivity with bromobenzene or 1-bromoadamantane even after being heated for several days at 70 °C. All new complexes were characterized by conventional spectroscopic and analytical methods, and the solid-state molecular structures of most of them were established by single-crystal X-ray crystallographic analyses.
中文翻译:

的1,2-双(二甲基膦基)乙烷(DMPE)配位体中的Cp *沫的Hemilability(NO)(κ 2 -dmpe)
的Cp *沫的反应(NO)氯2与1当量的1,2-双(二甲基膦基)乙烷(DMPE)在THF中在环境温度下的形式的[Cp *沫(NO)(CL)(κ的2 -dmpe)]氯(1),其可分离为分析纯黄色粉末,产率为65%。2当量Cp的的进一步添加2 Co而1在CH 2氯2次,得到暗红色的Cp *沫(NO)(κ 2 -dmpe)(2),将其在36%的产率分离通过重结晶从Et 2在-O - 30°C。的苯溶液反应2与在直接生产深蓝色(μ-S)的[Cp *沫(NO)的元素硫的结果等摩尔量(κ 1-dmpeS)] 2(3),这是仅由一个硫原子桥接并涉及Mo═S═Mo键的双金属过渡金属配合物的罕见例子。相比之下,反应2与苯结果过量硫的地层中的Cp *沫的(NO)(η 2 -S 2)(κ 1 -dmpeS)(4)。还可通过向3中添加元素硫来形成配合物4,从而表明3是4的前体。的Cp *沫(NO)(κ 2 -dmpe)(2)在用有机溴化物处理时也经历了有趣的转变。例如,2与5当量苄基溴在THF中的反应可能在70℃下通过自由基中间体产生双金属配合物(μ-dmpe)[Cp * Mo(NO)Br 2 ] 2(5)和联苄。相对于其与苄基溴反应,配合物2点的形式[沫(NO)溴2(κ 2 -dmpe)] 2(6),烯烃,烷烃,1和Cp * H当与5当量1-溴丙烷或1的处理过的-溴辛烷在THF中的溶液在70°C下反应72小时。有趣的是,复杂2即使在70°C下加热几天,也不会与溴苯或1-溴金刚烷反应。所有新的配合物都通过常规的光谱学和分析方法进行了表征,其中大多数的固态分子结构是通过单晶X射线晶体学分析确定的。
更新日期:2017-09-06
中文翻译:

的1,2-双(二甲基膦基)乙烷(DMPE)配位体中的Cp *沫的Hemilability(NO)(κ 2 -dmpe)
的Cp *沫的反应(NO)氯2与1当量的1,2-双(二甲基膦基)乙烷(DMPE)在THF中在环境温度下的形式的[Cp *沫(NO)(CL)(κ的2 -dmpe)]氯(1),其可分离为分析纯黄色粉末,产率为65%。2当量Cp的的进一步添加2 Co而1在CH 2氯2次,得到暗红色的Cp *沫(NO)(κ 2 -dmpe)(2),将其在36%的产率分离通过重结晶从Et 2在-O - 30°C。的苯溶液反应2与在直接生产深蓝色(μ-S)的[Cp *沫(NO)的元素硫的结果等摩尔量(κ 1-dmpeS)] 2(3),这是仅由一个硫原子桥接并涉及Mo═S═Mo键的双金属过渡金属配合物的罕见例子。相比之下,反应2与苯结果过量硫的地层中的Cp *沫的(NO)(η 2 -S 2)(κ 1 -dmpeS)(4)。还可通过向3中添加元素硫来形成配合物4,从而表明3是4的前体。的Cp *沫(NO)(κ 2 -dmpe)(2)在用有机溴化物处理时也经历了有趣的转变。例如,2与5当量苄基溴在THF中的反应可能在70℃下通过自由基中间体产生双金属配合物(μ-dmpe)[Cp * Mo(NO)Br 2 ] 2(5)和联苄。相对于其与苄基溴反应,配合物2点的形式[沫(NO)溴2(κ 2 -dmpe)] 2(6),烯烃,烷烃,1和Cp * H当与5当量1-溴丙烷或1的处理过的-溴辛烷在THF中的溶液在70°C下反应72小时。有趣的是,复杂2即使在70°C下加热几天,也不会与溴苯或1-溴金刚烷反应。所有新的配合物都通过常规的光谱学和分析方法进行了表征,其中大多数的固态分子结构是通过单晶X射线晶体学分析确定的。































 京公网安备 11010802027423号
京公网安备 11010802027423号