当前位置:
X-MOL 学术
›
Nat. Commun.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Cre/lox-assisted non-invasive in vivo tracking of specific cell populations by positron emission tomography.
Nature Communications ( IF 14.7 ) Pub Date : 2017-09-05 , DOI: 10.1038/s41467-017-00482-y Martin Thunemann , Barbara F. Schörg , Susanne Feil , Yun Lin , Jakob Voelkl , Matthias Golla , Angelos Vachaviolos , Ursula Kohlhofer , Leticia Quintanilla-Martinez , Marcus Olbrich , Walter Ehrlichmann , Gerald Reischl , Christoph M. Griessinger , Harald F. Langer , Meinrad Gawaz , Florian Lang , Michael Schäfers , Manfred Kneilling , Bernd J. Pichler , Robert Feil
Nature Communications ( IF 14.7 ) Pub Date : 2017-09-05 , DOI: 10.1038/s41467-017-00482-y Martin Thunemann , Barbara F. Schörg , Susanne Feil , Yun Lin , Jakob Voelkl , Matthias Golla , Angelos Vachaviolos , Ursula Kohlhofer , Leticia Quintanilla-Martinez , Marcus Olbrich , Walter Ehrlichmann , Gerald Reischl , Christoph M. Griessinger , Harald F. Langer , Meinrad Gawaz , Florian Lang , Michael Schäfers , Manfred Kneilling , Bernd J. Pichler , Robert Feil
|
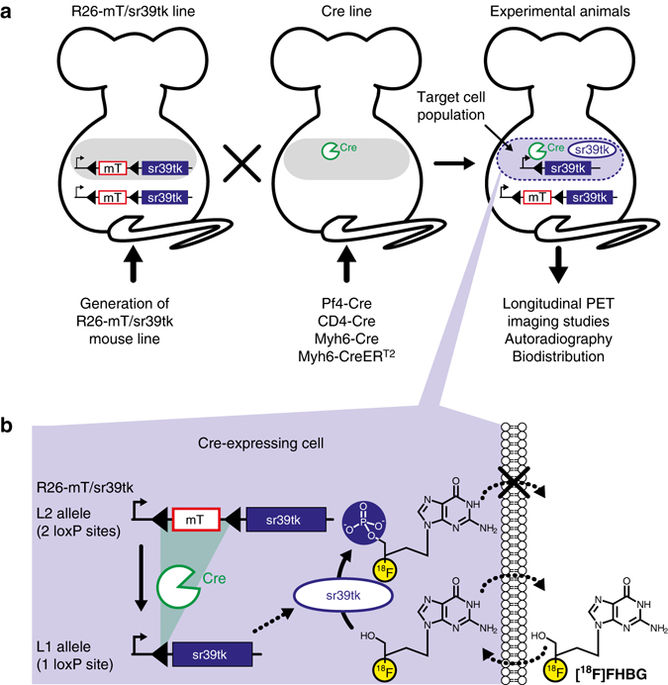
Many pathophysiological processes are associated with proliferation, migration or death of distinct cell populations. Monitoring specific cell types and their progeny in a non-invasive, longitudinal and quantitative manner is still challenging. Here we show a novel cell-tracking system that combines Cre/lox-assisted cell fate mapping with a thymidine kinase (sr39tk) reporter gene for cell detection by positron emission tomography (PET). We generate Rosa26-mT/sr39tk PET reporter mice and induce sr39tk expression in platelets, T lymphocytes or cardiomyocytes. As proof of concept, we demonstrate that our mouse model permits longitudinal PET imaging and quantification of T-cell homing during inflammation and cardiomyocyte viability after myocardial infarction. Moreover, Rosa26-mT/sr39tk mice are useful for whole-body characterization of transgenic Cre mice and to detect previously unknown Cre activity. We anticipate that the Cre-switchable PET reporter mice will be broadly applicable for non-invasive long-term tracking of selected cell populations in vivo.Non-invasive cell tracking is a powerful method to visualize cells in vivo under physiological and pathophysiological conditions. Here Thunemann et al. generate a mouse model for in vivo tracking and quantification of specific cell types by combining a PET reporter gene with Cre-dependent activation that can be exploited for any cell population for which a Cre mouse line is available.
中文翻译:

Cre / lox辅助通过正电子发射断层扫描对特定细胞群体进行无创体内跟踪。
许多病理生理过程与不同细胞群的增殖,迁移或死亡有关。以无创,纵向和定量方式监测特定细胞类型及其后代仍然具有挑战性。在这里,我们展示了一种新型的细胞跟踪系统,该系统结合了Cre / lox辅助的细胞命运定位与胸腺嘧啶激酶(sr39tk)报告基因,可通过正电子发射断层扫描(PET)检测细胞。我们生成Rosa26-mT / sr39tk PET报告基因小鼠,并诱导sr39tk在血小板,T淋巴细胞或心肌细胞中的表达。作为概念验证,我们证明了我们的小鼠模型允许在炎症和心肌梗死后心肌细胞活力期间进行纵向PET成像和T细胞归巢量化。而且,Rosa26-mT / sr39tk小鼠可用于全身表征转基因Cre小鼠,并检测先前未知的Cre活性。我们预期Cre可切换PET报告基因小鼠将广泛应用于体内非侵入性长期跟踪选定的细胞群。非侵入性细胞跟踪是一种在生理和病理生理条件下可视化体内细胞的强大方法。在这里Thunemann等。通过将PET报告基因与Cre依赖性激活相结合,可生成可用于体内跟踪和定量特定细胞类型的小鼠模型,该模型可用于可使用Cre小鼠系的任何细胞群体。我们预期Cre可切换PET报告基因小鼠将广泛应用于体内非侵入性长期跟踪选定的细胞群。非侵入性细胞跟踪是一种在生理和病理生理条件下可视化体内细胞的强大方法。在这里Thunemann等。通过将PET报告基因与Cre依赖性激活相结合,可生成可用于体内跟踪和定量特定细胞类型的小鼠模型,该模型可用于可使用Cre小鼠系的任何细胞群体。我们预期Cre可切换的PET报告基因小鼠将广泛应用于体内选择的细胞群体的非侵入性长期跟踪。非侵入性细胞跟踪是一种在生理和病理生理条件下可视化体内细胞的强大方法。在这里Thunemann等。通过将PET报告基因与Cre依赖性激活相结合,可生成可用于体内跟踪和定量特定细胞类型的小鼠模型,该模型可用于可使用Cre小鼠系的任何细胞群体。
更新日期:2017-09-05
中文翻译:

Cre / lox辅助通过正电子发射断层扫描对特定细胞群体进行无创体内跟踪。
许多病理生理过程与不同细胞群的增殖,迁移或死亡有关。以无创,纵向和定量方式监测特定细胞类型及其后代仍然具有挑战性。在这里,我们展示了一种新型的细胞跟踪系统,该系统结合了Cre / lox辅助的细胞命运定位与胸腺嘧啶激酶(sr39tk)报告基因,可通过正电子发射断层扫描(PET)检测细胞。我们生成Rosa26-mT / sr39tk PET报告基因小鼠,并诱导sr39tk在血小板,T淋巴细胞或心肌细胞中的表达。作为概念验证,我们证明了我们的小鼠模型允许在炎症和心肌梗死后心肌细胞活力期间进行纵向PET成像和T细胞归巢量化。而且,Rosa26-mT / sr39tk小鼠可用于全身表征转基因Cre小鼠,并检测先前未知的Cre活性。我们预期Cre可切换PET报告基因小鼠将广泛应用于体内非侵入性长期跟踪选定的细胞群。非侵入性细胞跟踪是一种在生理和病理生理条件下可视化体内细胞的强大方法。在这里Thunemann等。通过将PET报告基因与Cre依赖性激活相结合,可生成可用于体内跟踪和定量特定细胞类型的小鼠模型,该模型可用于可使用Cre小鼠系的任何细胞群体。我们预期Cre可切换PET报告基因小鼠将广泛应用于体内非侵入性长期跟踪选定的细胞群。非侵入性细胞跟踪是一种在生理和病理生理条件下可视化体内细胞的强大方法。在这里Thunemann等。通过将PET报告基因与Cre依赖性激活相结合,可生成可用于体内跟踪和定量特定细胞类型的小鼠模型,该模型可用于可使用Cre小鼠系的任何细胞群体。我们预期Cre可切换的PET报告基因小鼠将广泛应用于体内选择的细胞群体的非侵入性长期跟踪。非侵入性细胞跟踪是一种在生理和病理生理条件下可视化体内细胞的强大方法。在这里Thunemann等。通过将PET报告基因与Cre依赖性激活相结合,可生成可用于体内跟踪和定量特定细胞类型的小鼠模型,该模型可用于可使用Cre小鼠系的任何细胞群体。













































 京公网安备 11010802027423号
京公网安备 11010802027423号